Distinctive microRNA signature of acute myeloid leukemia bearing cytoplasmic mutated nucleophosmin
- PMID: 18308931
- PMCID: PMC2268779
- DOI: 10.1073/pnas.0800135105
Distinctive microRNA signature of acute myeloid leukemia bearing cytoplasmic mutated nucleophosmin
Erratum in
-
Correction for Garzon et al., Distinctive microRNA signature of acute myeloid leukemia bearing cytoplasmic mutated nucleophosmin.Proc Natl Acad Sci U S A. 2024 Sep 3;121(36):e2415544121. doi: 10.1073/pnas.2415544121. Epub 2024 Aug 30. Proc Natl Acad Sci U S A. 2024. PMID: 39213182 Free PMC article. No abstract available.
-
Correction for Garzon et al., Distinctive microRNA signature of acute myeloid leukemia bearing cytoplasmic mutated nucleophosmin.Proc Natl Acad Sci U S A. 2024 Oct 22;121(43):e2419360121. doi: 10.1073/pnas.2419360121. Epub 2024 Oct 18. Proc Natl Acad Sci U S A. 2024. PMID: 39423245 Free PMC article. No abstract available.
Abstract
Acute myeloid leukemia (AML) carrying NPM1 mutations and cytoplasmic nucleophosmin (NPMc+ AML) accounts for about one-third of adult AML and shows distinct features, including a unique gene expression profile. MicroRNAs (miRNAs) are small noncoding RNAs of 19-25 nucleotides in length that have been linked to the development of cancer. Here, we investigated the role of miRNAs in the biology of NPMc+ AML. The miRNA expression was evaluated in 85 adult de novo AML patients characterized for subcellular localization/mutation status of NPM1 and FLT3 mutations using a custom microarray platform. Data were analyzed by using univariate t test within BRB tools. We identified a strong miRNA signature that distinguishes NPMc+ mutated (n = 55) from the cytoplasmic-negative (NPM1 unmutated) cases (n = 30) and includes the up-regulation of miR-10a, miR-10b, several let-7 and miR-29 family members. Many of the down-regulated miRNAs including miR-204 and miR-128a are predicted to target several HOX genes. Indeed, we confirmed that miR-204 targets HOXA10 and MEIS1, suggesting that the HOX up-regulation observed in NPMc+ AML may be due in part by loss of HOX regulators-miRNAs. FLT3-ITD+ samples were characterized by up-regulation of miR-155. Further experiments demonstrated that the up-regulation of miR-155 was independent from FLT3 signaling. Our results identify a unique miRNA signature associated with NPMc+ AML and provide evidence that support a role for miRNAs in the regulation of HOX genes in this leukemia subtype. Moreover, we found that miR-155 was strongly but independently associated with FLT3-ITD mutations.
Conflict of interest statement
B.F. and C.M. have applied for a patent on clinical use of NPM1 mutants. The authors declare no other conflict of interest.
Figures


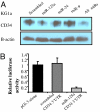

References
-
- Lowenberg B, Downing JR, Burnett A. Acute Myeloid leukemia. N Engl J Med. 1999;341:1051–1062. - PubMed
-
- Burnett AK. Current controversies: Which patients with acute myeloid leukemia should receive bone marrow transplantation? An adult theater's view. Br J Haematol. 2002;118:357–364. - PubMed
-
- Kiyoi H, et al. Prognostic implication of FLT3 and N-RAS gene mutations in acute myeloid leukemia. Blood. 1999;93:3074–3080. - PubMed
-
- Shih LY, et al. Internal tandem duplication of FLT3 in relapsed acute myeloid leukemia: A comparative analysis of bone marrow samples from 108 adult patients at diagnosis and relapse. Blood. 2000;100:2387–2392. - PubMed
-
- Frohling S, et al. CEBPA mutations in younger adults with acute myeloid leukemia and normal cytogenetics: Prognostic relevance and analysis of cooperating mutations. J Clin Oncol. 2004;22:624–633. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

