Role of mitochondrial dysfunction in insulin resistance
- PMID: 18309108
- PMCID: PMC2963150
- DOI: 10.1161/CIRCRESAHA.107.165472
Role of mitochondrial dysfunction in insulin resistance
Abstract
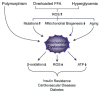
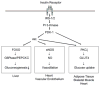
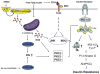
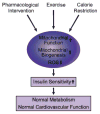
Insulin resistance is characteristic of obesity, type 2 diabetes, and components of the cardiometabolic syndrome, including hypertension and dyslipidemia, that collectively contribute to a substantial risk for cardiovascular disease. Metabolic actions of insulin in classic insulin target tissues (eg, skeletal muscle, fat, and liver), as well as actions in nonclassic targets (eg, cardiovascular tissue), help to explain why insulin resistance and metabolic dysregulation are central in the pathogenesis of the cardiometabolic syndrome and cardiovascular disease. Glucose and lipid metabolism are largely dependent on mitochondria to generate energy in cells. Thereby, when nutrient oxidation is inefficient, the ratio of ATP production/oxygen consumption is low, leading to an increased production of superoxide anions. Reactive oxygen species formation may have maladaptive consequences that increase the rate of mutagenesis and stimulate proinflammatory processes. In addition to reactive oxygen species formation, genetic factors, aging, and reduced mitochondrial biogenesis all contribute to mitochondrial dysfunction. These factors also contribute to insulin resistance in classic and nonclassic insulin target tissues. Insulin resistance emanating from mitochondrial dysfunction may contribute to metabolic and cardiovascular abnormalities and subsequent increases in cardiovascular disease. Furthermore, interventions that improve mitochondrial function also improve insulin resistance. Collectively, these observations suggest that mitochondrial dysfunction may be a central cause of insulin resistance and associated complications. In this review, we discuss mechanisms of mitochondrial dysfunction related to the pathophysiology of insulin resistance in classic insulin-responsive tissue, as well as cardiovascular tissue.
Figures


Similar articles
-
Perspectives and potential applications of mitochondria-targeted antioxidants in cardiometabolic diseases and type 2 diabetes.Med Res Rev. 2014 Jan;34(1):160-89. doi: 10.1002/med.21285. Epub 2013 May 3. Med Res Rev. 2014. PMID: 23650093 Review.
-
Mitochondrial biogenesis: pharmacological approaches.Curr Pharm Des. 2014;20(35):5507-9. doi: 10.2174/138161282035140911142118. Curr Pharm Des. 2014. PMID: 24606795
-
Cytosolic lipid excess-induced mitochondrial dysfunction is the cause or effect of high fat diet-induced skeletal muscle insulin resistance: a molecular insight.Mol Biol Rep. 2019 Feb;46(1):957-963. doi: 10.1007/s11033-018-4551-7. Epub 2018 Dec 8. Mol Biol Rep. 2019. PMID: 30535784 Review.
-
Mitochondrial biogenesis in the metabolic syndrome and cardiovascular disease.J Mol Med (Berl). 2010 Oct;88(10):993-1001. doi: 10.1007/s00109-010-0663-9. Epub 2010 Aug 20. J Mol Med (Berl). 2010. PMID: 20725711 Free PMC article. Review.
-
Role of Mitochondrial Dysfunction in Hypertension and Obesity.Curr Hypertens Rep. 2017 Feb;19(2):11. doi: 10.1007/s11906-017-0710-9. Curr Hypertens Rep. 2017. PMID: 28233236 Review.
Cited by
-
Hyperinsulinemia-induced vascular smooth muscle cell (VSMC) migration and proliferation is mediated by converging mechanisms of mitochondrial dysfunction and oxidative stress.Mol Cell Biochem. 2013 Jan;373(1-2):95-105. doi: 10.1007/s11010-012-1478-5. Epub 2012 Oct 17. Mol Cell Biochem. 2013. PMID: 23073711
-
Pivotal role of vitamin D in mitochondrial health, cardiac function, and human reproduction.EXCLI J. 2022 Jul 20;21:967-990. doi: 10.17179/excli2022-4935. eCollection 2022. EXCLI J. 2022. PMID: 36110560 Free PMC article. Review.
-
Inhibition of glutaminolysis restores mitochondrial function in senescent stem cells.Cell Rep. 2022 Nov 29;41(9):111744. doi: 10.1016/j.celrep.2022.111744. Cell Rep. 2022. PMID: 36450260 Free PMC article.
-
Mitochondria in White, Brown, and Beige Adipocytes.Stem Cells Int. 2016;2016:6067349. doi: 10.1155/2016/6067349. Epub 2016 Mar 17. Stem Cells Int. 2016. PMID: 27073398 Free PMC article. Review.
-
High fat diet-induced modifications in membrane lipid and mitochondrial-membrane protein signatures precede the development of hepatic insulin resistance in mice.Mol Metab. 2014 Nov 14;4(1):39-50. doi: 10.1016/j.molmet.2014.11.004. eCollection 2015 Jan. Mol Metab. 2014. PMID: 25685688 Free PMC article.
References
-
- Smith SC., Jr Multiple risk factors for cardiovascular disease and diabetes mellitus. Am J Med. 2007;120:S3–S11. - PubMed
-
- Cooper SA, Whaley-Connell A, Habibi J, Wei Y, Lastra G, Manrique CM, Stas S, Sowers JR. Renin-angiotensin-aldosterone system and oxidative stress in cardiovascular insulin resistance. Am J Physiol Heart Circ Physiol. 2007;293:H2009–H2023. - PubMed
-
- Sowers JR. Insulin resistance and hypertension. Am J Physiol Heart Circ Physiol. 2004;286:H1597–H1602. - PubMed
-
- Reaven GM, Chen YD. Insulin resistance, its consequences, and coronary heart disease. Must we choose one culprit? Circulation. 1996;93:1780–1783. - PubMed
-
- Stas S, Whaley-Connell A, Habibi J, Appesh L, Hayden MR, Karuparthi PR, Qazi M, Morris EM, Cooper SA, Link CD, Stump C, Hay M, Ferrario C, Sowers JR. Mineralocorticoid receptor blockade attenuates chronic overexpression of the renin-angiotensin-aldosterone system stimulation of reduced nicotinamide adenine dinucleotide phosphate oxidase and cardiac remodeling. Endocrinology. 2007;148:3773–3780. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

