Phase III trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: CALGB 9781
- PMID: 18309943
- PMCID: PMC5126644
- DOI: 10.1200/JCO.2007.12.9593
Phase III trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: CALGB 9781
Abstract
Purpose: The primary treatment modality for patients with carcinoma of the esophagus or gastroesophageal junction has been surgery, although primary radiation therapy with concurrent chemotherapy produces similar results. As both have curative potential, there has been great interest in the use of trimodality therapy. To this end, we compared survival, response, and patterns of failure of trimodality therapy to esophagectomy alone in patients with nonmetastatic esophageal cancer.
Patients and methods: Four hundred seventy-five eligible patients were planned for enrollment. Patients were randomly assigned to either esophagectomy with node dissection alone or cisplatin 100 mg/m(2) and fluorouracil 1,000 mg/m(2)/d for 4 days on weeks 1 and 5 concurrent with radiation therapy (50.4 Gy total: 1.8 Gy/fraction over 5.6 weeks) followed by esophagectomy with node dissection.
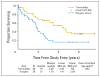
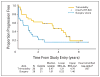
Results: Fifty-six patients were enrolled between October 1997 and March 2000, when the trial was closed due to poor accrual. Thirty patients were randomly assigned to trimodality therapy and 26 were assigned to surgery alone. Patient and tumor characteristics were similar between groups. Treatment was generally well tolerated. Median follow-up was 6 years. An intent-to-treat analysis showed a median survival of 4.48 v 1.79 years in favor of trimodality therapy (exact stratified log-rank, P = .002). Five-year survival was 39% (95% CI, 21% to 57%) v 16% (95% CI, 5% to 33%) in favor of trimodality therapy.
Conclusion: The results from this trial reflect a long-term survival advantage with the use of chemoradiotherapy followed by surgery in the treatment of esophageal cancer, and support trimodality therapy as a standard of care for patients with this disease.
Conflict of interest statement
Authors’ disclosures of potential conflicts of interest and author contributions are found at the end of this article. AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST The author(s) indicated no potential conflicts of interest.
Figures
Comment in
-
Neoadjuvant chemoradiotherapy in esophageal cancer: is it still the question?J Clin Oncol. 2008 Nov 1;26(31):5133-4; author reply 5134. doi: 10.1200/JCO.2008.19.0322. Epub 2008 Oct 6. J Clin Oncol. 2008. PMID: 18838699 No abstract available.
References
-
- Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11–20. - PubMed
-
- Macdonald JS, Smalley SR, Benedetti J, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med. 2001;345:725–730. - PubMed
-
- Bates BA, Detterbeck FC, Bernard SA, et al. Concurrent radiation therapy and chemotherapy followed by esophagectomy for localized esophageal carcinoma. J Clin Oncol. 1996;14:156–163. - PubMed
-
- Forastiere A, Orringer M, Perez-Tamayo C, et al. Preoperative chemoradiation followed by transhiatal esophagectomy for carcinoma of the esophagus: Final report. J Clin Oncol. 1993;11:1118–1123. - PubMed
-
- Kavanagh B, Anscher M, Leopold K, et al. Patterns of failure following combined modality therapy for esophageal cancer, 1984–1990. Int J Radiat Oncol Biol Phys. 1992;24:633–642. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

