Blockade of endothelinergic receptors prevents development of proliferative vitreoretinopathy in mice
- PMID: 18310504
- PMCID: PMC2276418
- DOI: 10.2353/ajpath.2008.070605
Blockade of endothelinergic receptors prevents development of proliferative vitreoretinopathy in mice
Abstract
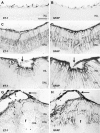
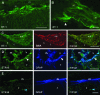
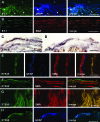
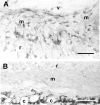
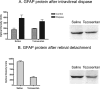
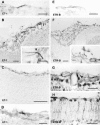
Proliferative vitreoretinopathy (PVR) is characterized by severe glial remodeling. Glial activation and proliferation that occur in brain diseases are modulated by endothelin-1 (ET-1) and its receptor B (ETR-B). Because retinal astrocytes contain ET-1 and express ETR-B, we studied the changes of these molecules in an experimental mouse model of PVR and in human PVR. Both ET-1 and ETR-B immunoreactivities increased in mouse retina after induction of PVR with dispase. Epi- and subretinal outgrowths also displayed these immunoreactivities in both human and experimental PVR. Additionally, myofibroblasts and other membranous cell types showed both ET-1 and ETR-B immunoreactivities. In early stages of experimentally induced PVR, prepro-ET-1 and ETR-B mRNA levels increased in the retina. These mRNA levels also increased after retinal detachment (RD) produced by subretinal injection. Treatment of mice with tezosentan, an antagonist of endothelinergic receptors, reduced the histopathological hallmarks of dispase-induced PVR: retinal folding, epiretinal outgrowth, and gliosis. Our findings in human and in dispase-induced PVR support the involvement of endothelinergic pathways in retinal glial activation and the phenotypic transformations that underlie the growth of membranes in this pathology. Elucidating these pathways further will help to develop pharmacological treatments to prevent PVR. In addition, the presence of ET-1 and ETR-B in human fibrous membranes suggests that similar treatments could be helpful after PVR has been established.
Figures
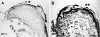


Similar articles
-
Immunoreactive ET-1 in the vitreous humor and epiretinal membranes of patients with proliferative vitreoretinopathy.Mol Vis. 2005 Jul 7;11:461-71. Mol Vis. 2005. PMID: 16030497
-
Changes in retinal gene expression in proliferative vitreoretinopathy: glial cell expression of HB-EGF.Mol Vis. 2005 Jun 10;11:397-413. Mol Vis. 2005. PMID: 15988409
-
Retina-specific expression of PDGF-B versus PDGF-A: vascular versus nonvascular proliferative retinopathy.Invest Ophthalmol Vis Sci. 2002 Jun;43(6):2001-6. Invest Ophthalmol Vis Sci. 2002. PMID: 12037011
-
Fibrotic Changes in Rhegmatogenous Retinal Detachment.Int J Mol Sci. 2025 Jan 25;26(3):1025. doi: 10.3390/ijms26031025. Int J Mol Sci. 2025. PMID: 39940795 Free PMC article. Review.
-
[Periostin in the Pathogenesis of Proliferative Vitreoretinopathy].Nippon Ganka Gakkai Zasshi. 2015 Nov;119(11):772-80. Nippon Ganka Gakkai Zasshi. 2015. PMID: 26685481 Review. Japanese.
Cited by
-
Knockdown of survivin results in inhibition of epithelial to mesenchymal transition in retinal pigment epithelial cells by attenuating the TGFβ pathway.Biochem Biophys Res Commun. 2018 Apr 6;498(3):573-578. doi: 10.1016/j.bbrc.2018.03.021. Epub 2018 Mar 6. Biochem Biophys Res Commun. 2018. PMID: 29522718 Free PMC article.
-
The inhibitory effect of small interference RNA protein kinase C-alpha on the experimental proliferative vitreoretinopathy induced by dispase in mice.Int J Nanomedicine. 2013;8:1563-72. doi: 10.2147/IJN.S37635. Epub 2013 Apr 19. Int J Nanomedicine. 2013. PMID: 23626468 Free PMC article.
-
Inflammation induces zebrafish regeneration.Neural Regen Res. 2021 Sep;16(9):1693-1701. doi: 10.4103/1673-5374.306059. Neural Regen Res. 2021. PMID: 33510057 Free PMC article. Review.
-
Endothelin, astrocytes and glaucoma.Exp Eye Res. 2011 Aug;93(2):170-7. doi: 10.1016/j.exer.2010.09.006. Epub 2010 Sep 16. Exp Eye Res. 2011. PMID: 20849847 Free PMC article. Review.
-
TNFα Induces Müller Glia to Transition From Non-proliferative Gliosis to a Regenerative Response in Mutant Zebrafish Presenting Chronic Photoreceptor Degeneration.Front Cell Dev Biol. 2019 Nov 26;7:296. doi: 10.3389/fcell.2019.00296. eCollection 2019. Front Cell Dev Biol. 2019. PMID: 31998714 Free PMC article.
References
-
- Charteris DG, Sethi CS, Lewis GP, Fisher SK. Proliferative vitreoretinopathy—developments in adjunctive treatment and retinal pathology. Eye. 2002;16:369–374. - PubMed
-
- Scott IU, Flynn HW, Jr, Murray TG, Feuer WJ. Outcomes of surgery for retinal detachment associated with proliferative vitreoretinopathy using perfluoro-n-octane: a multicenter study. Am J Ophthalmol. 2003;136:454–463. - PubMed
-
- Fisher SK, Lewis GP, Linberg KA, Verardo MR. Cellular remodeling in mammalian retina: results from studies of experimental retinal detachment. Prog Retin Eye Res. 2005;24:395–431. - PubMed
-
- Sethi CS, Lewis GP, Fisher SK, Leitner WP, Mann DL, Luthert PJ, Charteris DG. Glial remodeling and neural plasticity in human retinal detachment with proliferative vitreoretinopathy. Invest Ophthalmol Vis Sci. 2005;46:329–342. - PubMed
-
- Lewis GP, Fisher SK. Muller cell outgrowth after retinal detachment: association with cone photoreceptors. Invest Ophthalmol Vis Sci. 2000;41:1542–1545. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

