Lamin A-dependent misregulation of adult stem cells associated with accelerated ageing
- PMID: 18311132
- PMCID: PMC2396576
- DOI: 10.1038/ncb1708
Lamin A-dependent misregulation of adult stem cells associated with accelerated ageing
Abstract
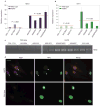
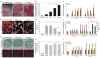
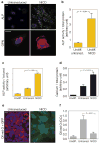
The premature-ageing disease Hutchinson-Gilford Progeria Syndrome (HGPS) is caused by constitutive production of progerin, a mutant form of the nuclear architectural protein lamin A. Progerin is also expressed sporadically in wild-type cells and has been linked to physiological ageing. Cells from HGPS patients exhibit extensive nuclear defects, including abnormal chromatin structure and increased DNA damage. At the organismal level, HGPS affects several tissues, particularly those of mesenchymal origin. How the cellular defects of HGPS cells lead to the organismal defects has been unclear. Here, we provide evidence that progerin interferes with the function of human mesenchymal stem cells (hMSCs). We find that expression of progerin activates major downstream effectors of the Notch signalling pathway. Induction of progerin in hMSCs changes their molecular identity and differentiation potential. Our results support a model in which accelerated ageing in HGPS patients, and possibly also physiological ageing, is the result of adult stem cell dysfunction and progressive deterioration of tissue functions.
Figures


References
-
- De Sandre-Giovannoli A, et al. Lamin A truncation in Hutchinson-Gilford progeria. Science. 2003;300:2055. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

