Antimalarial drugs inhibit human 5-HT(3) and GABA(A) but not GABA(C) receptors
- PMID: 18311193
- PMCID: PMC2438262
- DOI: 10.1038/bjp.2008.34
Antimalarial drugs inhibit human 5-HT(3) and GABA(A) but not GABA(C) receptors
Abstract
Background and purpose: Antimalarial compounds have been previously shown to inhibit rodent nicotinic acetylcholine (nACh) and 5-HT(3) receptors. Here, we extend these studies to include human 5-HT(3A), 5-HT(3AB), GABA(A) alpha1beta2, GABA(A) alpha1beta2gamma2 and GABA(C) rho1 receptors.
Experimental approach: We examined the effects of quinine, chloroquine and mefloquine on the electrophysiological properties of receptors expressed in Xenopus oocytes.
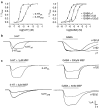
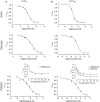
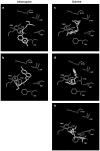
Key results: 5-HT(3A) receptor responses were inhibited by mefloquine, quinine and chloroquine with IC(50) values of 0.66, 1.06 and 24.3 microM. At 5-HT(3AB) receptors, the potencies of mefloquine (IC(50)=2.7 microM) and quinine (15.8 microM), but not chloroquine (23.6 microM), were reduced. Mefloquine, quinine and chloroquine had higher IC(50) values at GABA(A) alpha1beta2 (98.7, 0.40 and 0.46 mM, respectively) and GABA(A) alpha1beta2gamma2 receptors (0.38, 1.69 and 0.67 mM, respectively). No effect was observed at GABA(C) rho1 receptors. At all 5-HT(3) and GABA(A) receptors, chloroquine displayed competitive behaviour and mefloquine was non-competitive. Quinine was competitive at 5-HT(3A) and GABA(A) receptors, but non-competitive at 5-HT(3AB) receptors. Homology modelling in combination with automated docking suggested orientations of quinine and chloroquine at the GABA(A) receptor binding site.
Conclusions and implications: The effects of mefloquine, quinine and chloroquine are distinct at GABA(A) and GABA(C) receptors, whereas their effects on 5-HT(3AB) receptors are broadly similar to those at 5-HT(3A) receptors. IC(50) values for chloroquine and mefloquine at 5-HT(3) receptors are close to therapeutic blood concentrations required for malarial treatment, suggesting that their therapeutic use could be extended to include the treatment of 5-HT(3) receptor-related disorders.
Figures



Similar articles
-
The antimalarial drugs quinine, chloroquine and mefloquine are antagonists at 5-HT3 receptors.Br J Pharmacol. 2007 Jul;151(5):666-77. doi: 10.1038/sj.bjp.0707238. Epub 2007 May 14. Br J Pharmacol. 2007. PMID: 17502851 Free PMC article.
-
Inhibition of native 5-HT3 receptor-evoked contractions in guinea pig and mouse ileum by antimalarial drugs.Eur J Pharmacol. 2014 Sep 5;738:186-91. doi: 10.1016/j.ejphar.2014.05.043. Epub 2014 Jun 2. Eur J Pharmacol. 2014. PMID: 24886883
-
Effects of quinine, quinidine, and chloroquine on alpha9alpha10 nicotinic cholinergic receptors.Mol Pharmacol. 2005 Sep;68(3):822-9. doi: 10.1124/mol.105.014431. Epub 2005 Jun 13. Mol Pharmacol. 2005. PMID: 15955868
-
The antimalarial agent mefloquine inhibits ATP-sensitive K-channels.Br J Pharmacol. 2000 Oct;131(4):756-60. doi: 10.1038/sj.bjp.0703638. Br J Pharmacol. 2000. PMID: 11030725 Free PMC article.
-
Quinoline antimalarials: mechanisms of action and resistance.Int J Parasitol. 1997 Feb;27(2):231-40. doi: 10.1016/s0020-7519(96)00152-x. Int J Parasitol. 1997. PMID: 9088993 Review.
Cited by
-
Commonly Used Therapeutics Associated with Changes in Arousal Inhibit GABAAR Activation.Biomolecules. 2023 Feb 15;13(2):365. doi: 10.3390/biom13020365. Biomolecules. 2023. PMID: 36830736 Free PMC article.
-
The role of connexin-36 gap junctions in alcohol intoxication and consumption.Synapse. 2011 Aug;65(8):695-707. doi: 10.1002/syn.20885. Epub 2010 Dec 28. Synapse. 2011. PMID: 21638336 Free PMC article.
-
Effects of Quinine, Quinidine and Chloroquine on Human Muscle Nicotinic Acetylcholine Receptors.Front Pharmacol. 2018 Nov 20;9:1339. doi: 10.3389/fphar.2018.01339. eCollection 2018. Front Pharmacol. 2018. PMID: 30515099 Free PMC article.
-
Mefloquine effects on ventral tegmental area dopamine and GABA neuron inhibition: a physiologic role for connexin-36 GAP junctions.Synapse. 2011 Aug;65(8):804-13. doi: 10.1002/syn.20907. Epub 2011 Apr 7. Synapse. 2011. PMID: 21218452 Free PMC article.
-
A nondesensitizing kainate receptor point mutant.Mol Pharmacol. 2009 Sep;76(3):534-42. doi: 10.1124/mol.109.056598. Epub 2009 Jun 26. Mol Pharmacol. 2009. PMID: 19561126 Free PMC article.
References
-
- Adelusi SA, Salako LA. Tissue and blood concentrations of chloroquine following chronic administration in the rat. J Pharmacol. 1982;34:733–735. - PubMed
-
- Akabas MH. GABAA receptor structure–function studies: a reexamination in light of new acetylcholine receptor structures. Int Rev Neurobiol. 2004;62:1–43. - PubMed
-
- Ballestero JA, Plazas PV, Kracun S, Gomez-Casati ME, Taranda J, Rothlin CV, et al. Effects of quinine, quinidine, and chloroquine on α9α10 nicotinic cholinergic receptors. Mol Pharmacol. 2005;68:822–829. - PubMed
-
- Barann M, Dilger JP, Bonisch H, Gothert M, Dybek A, Urban BW. Inhibition of 5-HT3 receptors by propofol: equilibrium and kinetic measurements. Neuropharmacology. 2000;39:1064–1074. - PubMed
-
- Barann M, Gothert M, Bonisch H, Dybek A, Urban BW. 5-HT3 receptors in outside-out patches of N1E-115 neuroblastoma cells: basic properties and effects of pentobarbital. Neuropharmacology. 1997;36:655–664. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

