Inefficient presentation of tumor-derived antigen by tumor-infiltrating dendritic cells
- PMID: 18311487
- PMCID: PMC11029823
- DOI: 10.1007/s00262-008-0487-4
Inefficient presentation of tumor-derived antigen by tumor-infiltrating dendritic cells
Abstract
Background: Transplantable B16 melanoma is widely used as a tumor model to investigate tumor immunity. We wished to characterize the leukocyte populations infiltrating B16 melanoma tumors, and the functional properties of tumor-infiltrating dendritic cells (TIDC).
Materials and methods: We used the B16 melanoma cell line expressing ovalbumin protein (OVA) to investigate the phenotype and T cell stimulatory capacity of TIDC.
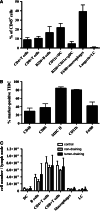
Results: The majority of leukocytes in B16 melanoma were macrophages, which colocalized with TIDCs, B and T cells to the peripheral area of the tumor. Both myeloid and plasmacytoid DC populations were present within tumors. Most of these DCs appeared immature, but about a third expressed a mature phenotype. TIDCs did not present tumor-derived antigen, as they were unable to induce the proliferation of tumor-specific CD4+ and CD8+ T cells in vitro unless in the presence of specific peptides. Some presentation of tumor-derived antigen could be demonstrated in the tumor-draining lymph node using in vivo proliferation assays. However, while proliferation of CD8+ T cells was reproducibly demonstrated, no proliferation of CD4+ T cells was observed.
Conclusion: In summary, our data suggest that DCs in tumors have limited antigen-presenting function. Inefficient antigen presentation extends to the tumor-draining lymph node, and may affect the generation of antitumor immune responses.
Figures



References
-
- Bell D, Chomarat P, Broyles D, Netto G, Harb GM, Lebecque S, Valladeau J, Davoust J, Palucka KA, Banchereau J. In breast carcinoma tissue, immature dendritic cells reside within the tumor, whereas mature dendritic cells are located in peritumoral areas. J Exp Med. 1999;190:1417–1426. doi: 10.1084/jem.190.10.1417. - DOI - PMC - PubMed
-
- Berthier-Vergnes O, Gaucherand M, Peguet-Navarro J, Plouet J, Pageaux JF, Schmitt D, Staquet MJ. Human melanoma cells inhibit the earliest differentiation steps of human Langerhans cell precursors but failed to affect the functional maturation of epidermal Langerhans cells. Br J Cancer. 2001;85:1944–1951. doi: 10.1054/bjoc.2001.2183. - DOI - PMC - PubMed
-
- Chiodoni C, Paglia P, Stoppacciaro A, Rodolfo M, Parenza M, Colombo MP. Dendritic cells infiltrating tumors cotransduced with granulocyte/macrophage colony-stimulating factor (GM-CSF) and CD40 ligand genes take up and present endogenous tumor-associated antigens, and prime naive mice for a cytotoxic T lymphocyte response. J Exp Med. 1999;190:125–133. doi: 10.1084/jem.190.1.125. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

