Interaction of asymmetric ABCC9-encoded nucleotide binding domains determines KATP channel SUR2A catalytic activity
- PMID: 18311911
- PMCID: PMC2743405
- DOI: 10.1021/pr7007847
Interaction of asymmetric ABCC9-encoded nucleotide binding domains determines KATP channel SUR2A catalytic activity
Abstract
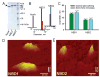
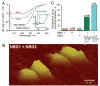
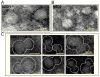
Nucleotide binding domains (NBDs) secure ATP-binding cassette (ABC) transporter function. Distinct from traditional ABC transporters, ABCC9-encoded sulfonylurea receptors (SUR2A) form, with Kir6.2 potassium channels, ATP-sensitive K+ (K ATP) channel complexes. SUR2A contains ATPase activity harbored within NBD2 and, to a lesser degree, NBD1, with catalytically driven conformations exerting determinate linkage on the Kir6.2 channel pore. While homodomain interactions typify NBDs of conventional ABC transporters, heterodomain NBD interactions and their functional consequence have not been resolved for the atypical SUR2A protein. Here, nanoscale protein topography mapped assembly of monodisperse purified recombinant SUR2A NBD1/NBD2 domains, precharacterized by dynamic light scattering. Heterodomain interaction produced conformational rearrangements inferred by secondary structural change in circular dichroism, and validated by atomic force and transmission electron microscopy. Physical engagement of NBD1 with NBD2 translated into enhanced intrinsic ATPase activity. Molecular modeling delineated a complemental asymmetry of NBD1/NBD2 ATP-binding sites. Mutation in the predicted catalytic base residue, D834E of NBD1, altered NBD1 ATPase activity disrupting potentiation of catalytic behavior in the NBD1/NBD2 interactome. Thus, NBD1/NBD2 assembly, resolved by a panel of proteomic approaches, provides a molecular substrate that determines the optimal catalytic activity in SUR2A, establishing a paradigm for the structure-function relationship within the K ATP channel complex.
Figures

References
-
- Dean M. The genetics of ATP-binding cassette transporters. Methods Enzymol. 2005;400:409–429. - PubMed
-
- Linton KJ. Structure and function of ABC transporters. Physiology (Bethesda) 2007;22:122–130. - PubMed
-
- Higgins CF, Linton KJ. The ATP switch model for ABC transporters. Nat Struct Mol Biol. 2004;11:918–926. - PubMed
-
- Biemans-Oldehinkel E, Doeven MK, Poolman B. ABC transporter architecture and regulatory roles of accessory domains. FEBS Lett. 2006;580:1023–1035. - PubMed
-
- Oram JF, Vaughan AM. ATP-Binding cassette cholesterol transporters and cardiovascular disease. Circ Res. 2006;99:1031–1043. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

