Oxidative metabolism of a fatty acid amide hydrolase-regulated lipid, arachidonoyltaurine
- PMID: 18311922
- PMCID: PMC2760074
- DOI: 10.1021/bi702530z
Oxidative metabolism of a fatty acid amide hydrolase-regulated lipid, arachidonoyltaurine
Abstract
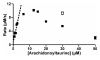
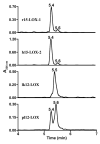
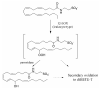
A novel class of lipids, N-acyltaurines, was recently discovered in fatty acid amide hydrolase knockout mice. In some peripheral tissues, such as liver and kidney, N-acyltaurines with long, polyunsaturated acyl chains are most prevalent. Polyunsaturated fatty acids are converted to a variety of signaling molecules by cyclooxygenases (COXs) and lipoxygenases (LOXs). The ability of COXs and LOXs to oxygenate arachidonoyltaurine was evaluated to gain insight into the potential metabolic fate of N-acyltaurines. Although arachidonoyltaurine was a poor substrate for COXs, mammalian 12 S- and 15 S-LOXs oxygenated arachidonoyltaurine with similar or better efficiency than arachidonic acid. Products of arachidonoyltaurine oxygenation were characterized by liquid chromatography-tandem mass spectrometry (LC-MS/MS). The positional specificity of single oxygenation was retained for 15 S-LOXs. However, platelet-type 12 S-LOX produced 12- and 15-hydroxyeicosatetraenoyltaurines (HETE-Ts). Furthermore, LOXs generated dihydroxyeicosatetraenoyltaurines (diHETE-Ts). Metabolism of arachidonoyltaurine by murine resident peritoneal macrophages (RPMs) was also profiled. Arachidonoyltaurine was rapidly taken up and converted primarily to 12-HETE-T. Over prolonged incubations, RPMs also generated small amounts of diHETE-T. Oxidative metabolism of polyunsaturated N-acyltaurines may represent a pathway for the generation or termination of novel signaling molecules.
Figures





References
-
- Guzman M. Cannabinoids: Potential anticancer agents. Nat. Rev. Cancer. 2003;3:745–755. - PubMed
-
- Karsak M, Cohen-Solal M, Freudenberg J, Ostertag A, Morieux C, Kornak U, Essig J, Erxlebe E, Bab I, Kubisch C, de Vernejoul M-C, Zimmer A. Cannabinoid receptor type 2 gene is associated with human osteoporosis. Hum. Mol. Genet. 2005;14:3389–3396. - PubMed
-
- Kathuria S, Gaetani S, Fegley D, Valino F, Duranti A, Tontini A, Mor M, Tarzia G, la Rana G, Calignano A, Giustino A, Tattoli M, Palmery M, Cuomo V, Piomelli D. Modulation of anxiety through blockade of anandamide hydrolysis. Nat. Med. 2003;9:76–81. - PubMed
-
- Kumar R, Chambers W, Pertwee R. Pharmacological actions and therapeutic uses of cannabis and cannabinoids. Anaesthesia. 2001;56:1059–1068. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

