Characterization of Trypanosoma brucei dihydroorotate dehydrogenase as a possible drug target; structural, kinetic and RNAi studies
- PMID: 18312275
- PMCID: PMC2877593
- DOI: 10.1111/j.1365-2958.2008.06131.x
Characterization of Trypanosoma brucei dihydroorotate dehydrogenase as a possible drug target; structural, kinetic and RNAi studies
Abstract
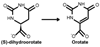
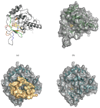
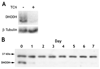
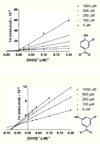
Nucleotide biosynthesis pathways have been reported to be essential in some protozoan pathogens. Hence, we evaluated the essentiality of one enzyme in the pyrimidine biosynthetic pathway, dihydroorotate dehydrogenase (DHODH) from the eukaryotic parasite Trypanosoma brucei through gene knockdown studies. RNAi knockdown of DHODH expression in bloodstream form T. brucei did not inhibit growth in normal medium, but profoundly retarded growth in pyrimidine-depleted media or in the presence of the known pyrimidine uptake antagonist 5-fluorouracil (5-FU). These results have significant implications for the development of therapeutics to combat T. brucei infection. Specifically, a combination therapy including a T. brucei-specific DHODH inhibitor plus 5-FU may prove to be an effective therapeutic strategy. We also show that this trypanosomal enzyme is inhibited by known inhibitors of bacterial Class 1A DHODH, in distinction to the sensitivity of DHODH from human and other higher eukaryotes. This selectivity is supported by the crystal structure of the T. brucei enzyme, which is reported here at a resolution of 1.95 A. Additional research, guided by the crystal structure described herein, is needed to identify potent inhibitors of T. brucei DHODH.
Figures







References
-
- Alexandrov A, Vignali M, LaCount DJ, Quartley E, de Vries C, Rosa DD, Babulski J, Mitchell SF, Schoenfeld LW, Fields S, Hol WG, Dumont ME, Phizicky EM, Grayhack EJ. A facile method for high-throughput co-expression of protein pairs. Mol Cell Proteomics. 2004;3:934–938. - PubMed
-
- Annoura T, Nara T, Makiuchi T, Hashimoto T, Aoki T. The origin of dihydroorotate dehydrogenase genes of kinetoplastids, with special reference to their biological significance and adaptation to anaerobic, parasitic conditions. J Mol Evol. 2005;60:113–127. - PubMed
-
- Baldwin J, Michnoff CH, Malmquist NA, White J, Roth MG, Rathod PK, Phillips MA. High-throughput screening for potent and selective inhibitors of Plasmodium falciparum dihy-droorotate dehydrogenase. J Biol Chem. 2005;280:21847–21853. - PubMed
-
- Beitz E. TEXshade: shading and labeling of multiple sequence alignments using LATEX2 epsilon. Bioinformatics. 2000;16:135–139. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

