Forkhead box transcription factor FOXO3a suppresses estrogen-dependent breast cancer cell proliferation and tumorigenesis
- PMID: 18312651
- PMCID: PMC2374977
- DOI: 10.1186/bcr1872
Forkhead box transcription factor FOXO3a suppresses estrogen-dependent breast cancer cell proliferation and tumorigenesis
Abstract
Introduction: Estrogen receptors (ERs) play key roles in breast cancer development and influence treatment outcome in breast cancer patients. Identification of molecules that regulate ER function may facilitate development of breast cancer treatment strategies. The forkhead box class O (FOXO) transcription factor FOXO3a has been suggested to function as a tumor suppressor in breast cancer. Using protein-protein interaction screening, we found that FOXO3a interacted with ER-alpha and ER-beta proteins in the human breast carcinoma cell line MCF-7, suggesting that there exists a crosstalk between the FOXO3a and ER signaling pathways in estrogen-dependent breast cancer cells.
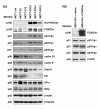
Methods: The interaction between FOXO3a and ER was investigated by using co-immunoprecipitation and immunoblotting assays. Inhibition of ER-alpha and ER-beta transactivation activity by FOXO was determined by luciferase reporter assays. Cell proliferation in culture was evaluated by counting cell numbers. Tumorigenesis was assessed in athymic mice that were injected with MCF-7 cell lines over-expressing FOXO3a. Protein expression levels of cyclin-dependent kinase inhibitors, cyclins, ERs, FOXM1, and the proteins encoded by ER-regulated genes in MCF-7 cell lines and breast tumors were examined by immunoblotting analysis and immunohistochemical staining.
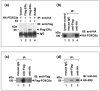
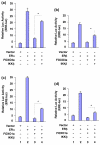
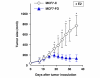
Results: We found that FOXO3a interacted with ER-alpha and ER-beta proteins and inhibited 17beta-estradiol (E2)-dependent, ER-regulated transcriptional activities. Consistent with these observations, expression of FOXO3a in the ER-positive MCF-7 cells decreased the expression of several ER-regulated genes, some of which play important roles in cell proliferation. Moreover, we found that FOXO3a upregulated the expression of the cyclin-dependent kinase inhibitors p21Cip1, p27Kip1, and p57Kip2. These findings suggest that FOXO3a induces cell growth arrest to effect tumor suppression. FOXO3a repressed the growth and survival of MCF-7 cells in cell culture. In an orthotopic breast cancer xenograft model in athymic mice, over-expression of FOXO3a in MCF-7 cells suppressed their E2-induced tumorigenesis, whereas knockdown of FOXO3a in MCF-7 resulted in the E2-independent growth.
Conclusion: Functional interaction between FOXO3a and ER plays a critical role in suppressing estrogen-dependent breast cancer cell growth and tumorigenesis in vivo. This suggests that agents that activate FOXO3a may be novel therapeutic agents that can inhibit and prevent tumor proliferation and development in breast cancer.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

