Human RFT1 deficiency leads to a disorder of N-linked glycosylation
- PMID: 18313027
- PMCID: PMC2427296
- DOI: 10.1016/j.ajhg.2007.12.021
Human RFT1 deficiency leads to a disorder of N-linked glycosylation
Abstract
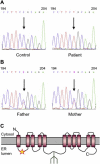
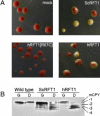
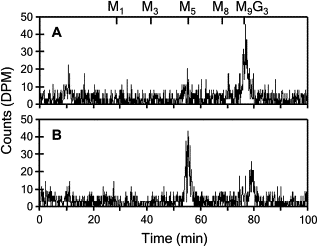
N-linked glycosylation is an essential posttranslational modification of proteins in eukaryotes. The substrate of N-linked glycosylation, dolichol pyrophosphate (DolPP)-GlcNAc(2)Man(9)Glc(3), is assembled through a complex series of ordered reactions requiring the translocation of the intermediate DolPP-GlcNAc(2)Man(5) structure across the endoplasmic-reticulum membrane. A young patient diagnosed with a congenital disorder of glycosylation characterized by an intracellular accumulation of DolPP-GlcNAc(2)Man(5) was found to carry a homozygous point mutation in the RFT1 gene. The c.199C-->T mutation introduced the amino acid substitution p.R67C. The human RFT1 protein shares 22% identity with its yeast ortholog, which is involved in the translocation of DolPP-GlcNAc(2)Man(5) from the cytosolic into the lumenal side of the endoplasmic reticulum. Despite the low sequence similarity between the yeast and the human RFT1 proteins, we demonstrated both their functional orthology and the pathologic effect of the human p.R67C mutation by complementation assay in Deltarft1 yeast cells. The causality of the RFT1 p.R67C mutation was further established by restoration of normal glycosylation profiles in patient-derived fibroblasts after lentiviral expression of a normal RFT1 cDNA. The definition of the RFT1 defect establishes the functional conservation of the DolPP-GlcNAc(2)Man(5) translocation process in eukaryotes. RFT1 deficiency in both yeast and human cells leads to the accumulation of incomplete DolPP-GlcNAc(2)Man(5) and to a profound glycosylation disorder in humans.
Figures
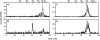

References
-
- Helenius A., Aebi M. Roles of N-linked glycans in the endoplasmic reticulum. Annu. Rev. Biochem. 2004;73:1019–1049. - PubMed
-
- Chantret I., Dancourt J., Dupre T., Delenda C., Bucher S., Vuillaumier-Barrot S., Ogier de Baulny H., Peletan C., Danos O., Seta N. A deficiency in dolichyl-P-glucose:Glc1Man9GlcNAc2-PP-dolichyl alpha3-glucosyltransferase defines a new subtype of congenital disorders of glycosylation. J. Biol. Chem. 2003;278:9962–9971. - PubMed
-
- Burda P., Aebi M. The dolichol pathway of N-linked glycosylation. Biochim. Biophys. Acta. 1999;1426:239–257. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

