Thiopurine analogues inhibit papain-like protease of severe acute respiratory syndrome coronavirus
- PMID: 18313035
- PMCID: PMC7092826
- DOI: 10.1016/j.bcp.2008.01.005
Thiopurine analogues inhibit papain-like protease of severe acute respiratory syndrome coronavirus
Abstract
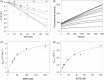
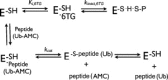
The papain-like protease of severe acute respiratory syndrome coronavirus (PLpro) (EC 3.4.22.46) is essential for the viral life cycle and therefore represents an important antiviral target. We have identified 6MP and 6TG as reversible and slow-binding inhibitors of SARS-CoV PLpro, which is the first report about small molecule reversible inhibitors of PLpro. The inhibition mechanism was investigated by kinetic measurements and computer docking. Both compounds are competitive, selective, and reversible inhibitors of the PLpro with K(is) values approximately 10 to 20 microM. A structure-function relationship study has identified the thiocarbonyl moiety of 6MP or 6TG as the active pharmacophore essential for these inhibitions, which has not been reported before. The inhibition is selective because these compounds do not exert significant inhibitory effects against other cysteine proteases, including SARS-CoV 3CLpro and several cathepsins. Thus, our results present the first potential chemical leads against SARS-CoV PLpro, which might be used as lead compounds for further optimization to enhance their potency against SARS-CoV. Both 6MP and 6TG are still used extensively in clinics, especially for children with acute lymphoblastic or myeloblastic leukemia. In light of the possible inhibition against subset of cysteine proteases, our study has emphasized the importance to study in depth these drug actions in vivo.
Figures



Similar articles
-
Thiopurine analogue inhibitors of severe acute respiratory syndrome-coronavirus papain-like protease, a deubiquitinating and deISGylating enzyme.Antivir Chem Chemother. 2009;19(4):151-6. doi: 10.1177/095632020901900402. Antivir Chem Chemother. 2009. PMID: 19374142
-
The SARS-coronavirus papain-like protease: structure, function and inhibition by designed antiviral compounds.Antiviral Res. 2015 Mar;115:21-38. doi: 10.1016/j.antiviral.2014.12.015. Epub 2014 Dec 29. Antiviral Res. 2015. PMID: 25554382 Free PMC article. Review.
-
Inhibitor recognition specificity of MERS-CoV papain-like protease may differ from that of SARS-CoV.ACS Chem Biol. 2015 Jun 19;10(6):1456-65. doi: 10.1021/cb500917m. Epub 2015 Mar 16. ACS Chem Biol. 2015. PMID: 25746232 Free PMC article.
-
Thiopurine analogs and mycophenolic acid synergistically inhibit the papain-like protease of Middle East respiratory syndrome coronavirus.Antiviral Res. 2015 Mar;115:9-16. doi: 10.1016/j.antiviral.2014.12.011. Epub 2014 Dec 24. Antiviral Res. 2015. PMID: 25542975 Free PMC article.
-
Quaternary structure, substrate selectivity and inhibitor design for SARS 3C-like proteinase.Curr Pharm Des. 2006;12(35):4555-64. doi: 10.2174/138161206779010396. Curr Pharm Des. 2006. PMID: 17168761 Review.
Cited by
-
Synergistic inhibitor binding to the papain-like protease of human SARS coronavirus: mechanistic and inhibitor design implications.ChemMedChem. 2013 Aug;8(8):1361-72. doi: 10.1002/cmdc.201300134. Epub 2013 Jun 20. ChemMedChem. 2013. PMID: 23788528 Free PMC article.
-
Broad-spectrum antivirals for the emerging Middle East respiratory syndrome coronavirus.J Infect. 2013 Dec;67(6):606-16. doi: 10.1016/j.jinf.2013.09.029. Epub 2013 Oct 3. J Infect. 2013. PMID: 24096239 Free PMC article.
-
Catalytic function and substrate specificity of the papain-like protease domain of nsp3 from the Middle East respiratory syndrome coronavirus.J Virol. 2014 Nov;88(21):12511-27. doi: 10.1128/JVI.01294-14. Epub 2014 Aug 20. J Virol. 2014. PMID: 25142582 Free PMC article.
-
Recent Advances in the Discovery of Deubiquitinating Enzyme Inhibitors.Prog Med Chem. 2016;55:149-92. doi: 10.1016/bs.pmch.2015.10.002. Epub 2016 Jan 12. Prog Med Chem. 2016. PMID: 26852935 Free PMC article. Review.
-
Viral deubiquitinating proteases and the promising strategies of their inhibition.Virus Res. 2024 Jun;344:199368. doi: 10.1016/j.virusres.2024.199368. Epub 2024 Apr 11. Virus Res. 2024. PMID: 38588924 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

