Extracellular matrix in the trabecular meshwork
- PMID: 18313051
- PMCID: PMC2376254
- DOI: 10.1016/j.exer.2008.01.013
Extracellular matrix in the trabecular meshwork
Abstract
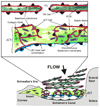
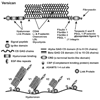
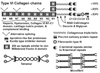
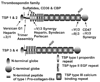
The extracellular matrix (ECM) of the trabecular meshwork (TM) is thought to be important in regulating intraocular pressure (IOP) in both normal and glaucomatous eyes. IOP is regulated primarily by a fluid resistance to aqueous humor outflow. However, neither the exact site nor the identity of the normal resistance to aqueous humor outflow has been established. Whether the site and nature of the increased outflow resistance, which is associated with open-angle glaucoma, is the same or different from the normal resistance is also unclear. The ECMs of the TM beams, juxtacanalicular region (JCT) and Schlemm's canal (SC) inner wall are comprised of fibrillar and non-fibrillar collagens, elastin-containing microfibrils, matricellular and structural organizing proteins, glycosaminoglycans (GAGs) and proteoglycans. Both basement membranes and stromal ECM are present in the TM beams and JCT region. Cell adhesion proteins, cell surface ECM receptors and associated binding proteins are also present in the beams, JCT and SC inner wall region. The outflow pathway ECM is relatively dynamic, undergoing constant turnover and remodeling. Regulated changes in enzymes responsible for ECM degradation and biosynthetic replacement are observed. IOP homeostasis, triggered by pressure changes or mechanical stretching of the TM, appears to involve ECM turnover. Several cytokines, growth factors and drugs, which affect the outflow resistance, change ECM component expression, mRNA alternative splicing, cellular cytoskeletal organization or all of these. Changes in ECM associated with open-angle glaucoma have been identified.
Figures




References
-
- Abu-Lail NI, Ohashi T, et al. Understanding the elasticity of fibronectin fibrils: unfolding strengths of FN-III and GFP domains measured by single molecule force spectroscopy. Matrix Biol. 2006;25:175–184. - PubMed
-
- Acott TS. Trabecular extracellular matrix regulation. In: Drance SM, Van Buskirk EM, Neufeld AH, editors. Pharmacology of Glaucoma. Baltimore: Williams & Wilkins; 1992. pp. 125–157.
-
- Acott TS. Biochemistry of aqueous humor outflow. In: Kaufman PL, Mittag TW, editors. Textbook of Ophthalmology. Vol. 7. London: Mosby; 1994a. pp. 1.47–1.78.
-
- Acott TS. Receptor biology and glaucoma - Integrins in the eye. In: Anderson DR, Drance SM, editors. Encounters in glaucoma research 1: Receptor biology and glaucoma. Milano: Foglizza Editore; 1994b. pp. 97–135.
-
- Acott TS, Kingsley PD, et al. Human trabecular meshwork organ culture: Morphology and glycosaminoglycan synthesis. Invest Ophthalmol Vis Sci. 1988;29:90–100. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

