Gain of function in IKs secondary to a mutation in KCNE5 associated with atrial fibrillation
- PMID: 18313602
- PMCID: PMC2515863
- DOI: 10.1016/j.hrthm.2007.12.019
Gain of function in IKs secondary to a mutation in KCNE5 associated with atrial fibrillation
Abstract
Background: Atrial fibrillation (AF) is the most common clinical arrhythmia and a major cause of cardiovascular morbidity and mortality. Among the gene defects previously associated with AF is a gain of function of the slowly activating delayed rectifier potassium current IKs, secondary to mutations in KCNQ1. Coexpression of KCNE5, the gene encoding the MiRP4 beta-subunit, has been shown to reduce IKs.
Objective: The purpose of this study was to test the hypothesis that mutations in KCNE5 are associated with AF in a large cohort of patients with AF.
Methods: One-hundred fifty-eight patients with AF were screened for mutations in the coding region of KCNE5.
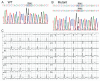
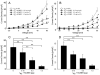
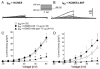
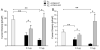
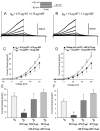
Results: A missense mutation involving substitution of a phenylalanine for leucine at position 65 (L65F) was identified in one patient. This patient did not have a history of familial AF, and neither KCNQ1 nor KCNE2 mutations were found. Transient transfection of Chinese hamster ovary (CHO) cells expressing IKs(KCNQ1+KCNE1) with KCNE5 suppressed the developing and tail currents of IKs in a concentration-dependent manner. Transient transfection with KCNE5-L65F failed to suppress IKs, yielding a current indistinguishable from that recorded in the absence of KCNE5. Developing currents recorded during a test pulse to +60 mV and tail currents recorded upon repolarization to -40 mV both showed a significant concentration-dependent gain of function in IKs with expression of KCNE5-L65F vs KCNE5-WT.
Conclusion: The results of this study suggest that a missense mutation in KCNE5 may be associated with nonfamilial or acquired forms of AF. The arrhythmogenic mechanism most likely is a gain of function of IKs.
Figures

Comment on
-
Ion channel mutations in AF: signal or noise?Heart Rhythm. 2008 Mar;5(3):436-7. doi: 10.1016/j.hrthm.2008.01.014. Epub 2008 Jan 17. Heart Rhythm. 2008. PMID: 18313603 Free PMC article. No abstract available.
References
-
- Frost L, Engholm G, Moller H, et al. Decrease in mortality in patients with a hospital diagnosis of atrial fibrillation in Denmark during the period 1980-1993. Eur Heart J. 1999;20:1592–1599. - PubMed
-
- Friberg J, Scharling H, Gadsboll N, et al. Sex-specific increase in the prevalence of atrial fibrillation (The Copenhagen City Heart Study) Am J Cardiol. 2003;92:1419–1423. - PubMed
-
- Frost L, Engholm G, Johnsen S, et al. Incident stroke after discharge from the hospital with a diagnosis of atrial fibrillation. Am J Med. 2000;108:36–40. - PubMed
-
- Grogan M, Smith HC, Gersh BJ, et al. Left ventricular dysfunction due to atrial fibrillation in patients initially believed to have idiopathic dilated cardiomyopathy. Am J Cardiol. 1992;69:1570–1573. - PubMed
-
- Darbar D, Herron KJ, Ballew JD, et al. Familial atrial fibrillation is a genetically heterogeneous disorder. J Am Coll Cardiol. 2003;41:2185–2192. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

