Ventricular-vascular interaction in heart failure
- PMID: 18313622
- PMCID: PMC2586173
- DOI: 10.1016/j.hfc.2007.10.001
Ventricular-vascular interaction in heart failure
Abstract
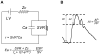
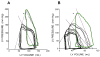
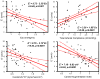
Nearly half of all patients who have heart failure have preserved ejection fraction (HFpEF). Patients who have HFpEF tend to be older, female, and hypertensive, and characteristically display increased ventricular and arterial stiffening. In this article, we discuss the pathophysiology of abnormal ventriculoarterial stiffening and how it affects ventricular function, cardiovascular hemodynamics, reserve capacity, and symptoms. We conclude by exploring how novel treatment strategies targeting abnormal ventricular-arterial interaction might prove useful in the treatment of patients who have HFpEF.
Figures



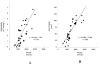
Republished in
-
Ventricular-vascular interaction in heart failure.Cardiol Clin. 2011 Aug;29(3):447-59. doi: 10.1016/j.ccl.2011.06.004. Cardiol Clin. 2011. PMID: 21803232
References
-
- Suga H, Sagawa K. Instantaneous pressure-volume relationships and their ratio in the excised, supported canine left ventricle. Circ Res Jul. 1974;35(1):117–126. - PubMed
-
- Kass DA, Kelly RP. Ventriculo-arterial coupling: concepts, assumptions, and applications. Ann Biomed Eng. 1992;20(1):41–62. - PubMed
-
- Milnor WR. Arterial impedance as ventricular afterload. Circ Res May. 1975;36(5):565–570. - PubMed
-
- Murgo JP, Westerhof N, Giolma JP, Altobelli SA. Aortic input impedance in normal man: relationship to pressure wave forms. Circulation Jul. 1980;62(1):105–116. - PubMed
-
- Sunagawa K, Maughan WL, Burkhoff D, Sagawa K. Left ventricular interaction with arterial load studied in isolated canine ventricle. Am J Physiol Nov. 1983;245(5 Pt 1):H773–780. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

