Cofactor biosynthesis--still yielding fascinating new biological chemistry
- PMID: 18314013
- PMCID: PMC2677635
- DOI: 10.1016/j.cbpa.2008.02.006
Cofactor biosynthesis--still yielding fascinating new biological chemistry
Abstract
This mini review covers recent advances in the mechanistic enzymology of cofactor biosynthesis.
Figures
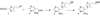
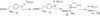


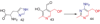



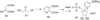
References
-
-
Hanes JW, Ealick SE, Begley TP. Thiamin Phosphate Synthase: The Rate of Pyrimidine Carbocation Formation. Journal of the American Chemical Society. 2007;129:4860–4861. Structural and kinetic characterization of the pyrimidine carbocation intermediate generated at the active site of thiamin phosphate synthase
-
-
-
Chatterjee A, Han X, McLafferty FW, Begley TP. Biosynthesis of thiamin thiazole: determination of the regiochemistry of the S/O acyl shift by using 1,4-dideoxy-D-xylulose-5-phosphate. Angewandte Chemie, International Edition. 2006;45:3507–3510. Determination that the acyl shift in thiamin thiazole biosynthesis occurs to the C3 hydroxyl of DXP
-
-
-
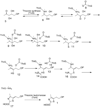
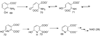
Dorrestein PC, Zhai H, McLafferty FW, Begley TP. The biosynthesis of the thiazole phosphate moiety of thiamin: the sulfur transfer mediated by the sulfur carrier protein ThiS. Chemistry & Biology. 2004;11:1373–1381. Mechanistic characterization of the bacterial thiazole synthase
-
-
-
Dorrestein PC, Zhai H, Taylor SV, McLafferty FW, Begley TP. The biosynthesis of the thiazole phosphate moiety of thiamin (vitamin B1): the early steps catalyzed by thiazole synthase. Journal of the American Chemical Society. 2004;126:3091–3096. Mechanistic characterization of the early steps in bacterial thiazole biosynthesis.
-
-
-
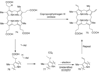
Duda DM, Walden H, Sfondouris J, Schulman BA. Structural analysis of Escherichia coli ThiF. Journal of Molecular Biology. 2005;349:774–786. The structure of the enzyme involved in the activation of the sulfur carrier protein involved in thiazole biosynthesis
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

