DETANO and nitrated lipids increase chloride secretion across lung airway cells
- PMID: 18314534
- PMCID: PMC2542453
- DOI: 10.1165/rcmb.2008-0005OC
DETANO and nitrated lipids increase chloride secretion across lung airway cells
Abstract
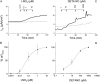
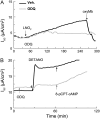
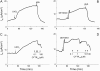
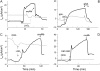
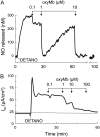
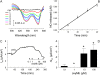
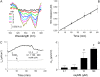
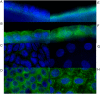
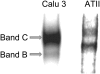
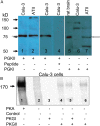
We investigated the cellular mechanisms by which nitric oxide (NO) increases chloride (Cl-) secretion across lung epithelial cells in vitro and in vivo. Addition of (Z)-1-[2-(2-aminoethyl)-N-(2-ammonioethyl) amino] diazen-1-ium-1, 2-diolate (DETANONOate [DETANO];1-1,000 microM) into apical compartments of Ussing chambers containing Calu-3 cells increased short-circuit currents (I(sc)) from 5.2 +/- 0.8 to 15.0 +/- 2.1 microA/cm(2) (X +/- 1 SE; n = 7; P < 0.001). NO generated from two nitrated lipids (nitrolinoleic and nitrooleic acids; 1-10 microM) also increased I(sc) by about 100%. Similar effects were noted across basolaterally, but not apically, permeabilized Calu-3 cells. None of these NO donors increased I(sc) in Calu-3 cells pretreated with 10 microM 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (an inhibitor of soluble guanylyl cyclase). Scavenging of NO either prevented or reversed the increase of I(sc). These data indicate that NO stimulation of soluble guanylyl cyclase was sufficient and necessary for the increase of I(sc) via stimulation of the apical cystic fibrosis transmembrane regulator (CFTR). Both Calu-3 and alveolar type II (ATII) cells contained CFTR, as demonstrated by in vitro phosphorylation of immunoprecipitated CFTR by protein kinase (PK) A. PKGII (but not PKGI) phosphorylated CFTR immuniprecipitated from Calu-3 cells. Corresponding values in ATII cells were below the threshold of detection. Furthermore, DETANO, 8-Br-cGMP, or 8-(4-chlorophenylthio)-cGMP (up to 2 mM each) did not increase Cl- secretion across amiloride-treated ATII cells in vitro. Measurements of nasal potential differences in anesthetized mice showed that perfusion of the nares with DETANO activated glybenclamide-sensitive Cl- secretion. These findings suggest that small concentrations of NO donors may prove beneficial in stimulating Cl- secretion across airway cells without promoting alveolar edema.
Figures


Similar articles
-
Reactive oxygen nitrogen species decrease cystic fibrosis transmembrane conductance regulator expression and cAMP-mediated Cl- secretion in airway epithelia.J Biol Chem. 2002 Nov 8;277(45):43041-9. doi: 10.1074/jbc.M203154200. Epub 2002 Aug 22. J Biol Chem. 2002. PMID: 12194970
-
Mechanisms of cystic fibrosis transmembrane conductance regulator activation by S-nitrosoglutathione.J Biol Chem. 2006 Apr 7;281(14):9190-9. doi: 10.1074/jbc.M513231200. Epub 2006 Jan 17. J Biol Chem. 2006. PMID: 16421103
-
Regulation of basolateral Cl(-) channels in airway epithelial cells: the role of nitric oxide.J Membr Biol. 2006;213(3):165-74. doi: 10.1007/s00232-006-0062-x. Epub 2007 Apr 28. J Membr Biol. 2006. PMID: 17468957
-
Nitric oxide inhibits highly selective sodium channels and the Na+/K+-ATPase in H441 cells.Am J Respir Cell Mol Biol. 2011 Jan;44(1):53-65. doi: 10.1165/2009-0335OC. Epub 2010 Feb 5. Am J Respir Cell Mol Biol. 2011. PMID: 20139350
-
C-type natriuretic peptide increases chloride permeability in normal and cystic fibrosis airway cells.Am J Respir Cell Mol Biol. 1997 Apr;16(4):464-70. doi: 10.1165/ajrcmb.16.4.9115758. Am J Respir Cell Mol Biol. 1997. PMID: 9115758
Cited by
-
Respiratory syncytial virus inhibits lung epithelial Na+ channels by up-regulating inducible nitric-oxide synthase.J Biol Chem. 2009 Mar 13;284(11):7294-306. doi: 10.1074/jbc.M806816200. Epub 2009 Jan 8. J Biol Chem. 2009. PMID: 19131335 Free PMC article.
-
p120-catenin expressed in alveolar type II cells is essential for the regulation of lung innate immune response.Am J Pathol. 2015 May;185(5):1251-63. doi: 10.1016/j.ajpath.2015.01.022. Epub 2015 Mar 13. Am J Pathol. 2015. PMID: 25773174 Free PMC article.
-
Participation of miR-200 in pulmonary fibrosis.Am J Pathol. 2012 Feb;180(2):484-93. doi: 10.1016/j.ajpath.2011.10.005. Epub 2011 Dec 20. Am J Pathol. 2012. PMID: 22189082 Free PMC article.
-
Role of epithelial sodium channels in the regulation of lung fluid homeostasis.Am J Physiol Lung Cell Mol Physiol. 2015 Dec 1;309(11):L1229-38. doi: 10.1152/ajplung.00319.2015. Epub 2015 Oct 2. Am J Physiol Lung Cell Mol Physiol. 2015. PMID: 26432872 Free PMC article. Review.
-
CPT-cGMP Is A New Ligand of Epithelial Sodium Channels.Int J Biol Sci. 2016 Jan 28;12(4):359-66. doi: 10.7150/ijbs.13764. eCollection 2016. Int J Biol Sci. 2016. PMID: 27019621 Free PMC article. Review.
References
-
- Schwiebert EM, Benos DJ, Egan ME, Stutts MJ, Guggino WB. CFTR is a conductance regulator as well as a chloride channel. Physiol Rev 1999;79:S145–S166. - PubMed
-
- Matalon S, O'Brodovich H. Sodium channels in alveolar epithelial cells: molecular characterization, biophysical properties, and physiological significance. Annu Rev Physiol 1999;61:627–661. - PubMed
-
- Schwiebert EM, Egan ME, Hwang TH, Fulmer SB, Allen SS, Cutting GR, Guggino WB. CFTR regulates outwardly rectifying chloride channels through an autocrine mechanism involving ATP. Cell 1995;81:1063–1073. - PubMed
-
- Zhu S, Yue G, Shoemaker RL, Matalon S. Adult alveolar type II cells lack cAMP and Ca2+-activated Cl− channels. Biochem Biophys Res Commun 1996;218:302–308. - PubMed
-
- Lazrak A, Thome U, Myles C, Ware J, Chen L, Venglarik CJ, Matalon S. cAMP regulation of Cl(−) and HCO(−)(3) secretion across rat fetal distal lung epithelial cells. Am J Physiol Lung Cell Mol Physiol 2002;282:L650–L658. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

