Alpha-1 adrenergic receptor transactivates signal transducer and activator of transcription-3 (Stat3) through activation of Src and epidermal growth factor receptor (EGFR) in hepatocytes
- PMID: 18314882
- PMCID: PMC2887298
- DOI: 10.1002/jcp.21420
Alpha-1 adrenergic receptor transactivates signal transducer and activator of transcription-3 (Stat3) through activation of Src and epidermal growth factor receptor (EGFR) in hepatocytes
Abstract
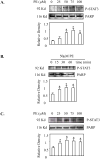
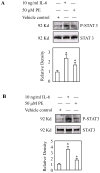
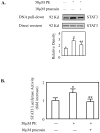
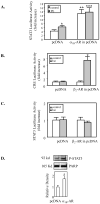
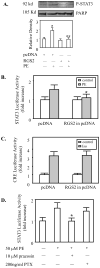
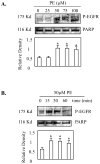
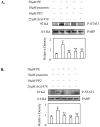
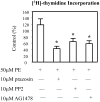
Hepatocytes express adrenergic receptors (ARs) that modulate several functions, including liver regeneration, hepatocyte proliferation, glycogenolysis, gluconeogenesis, synthesis of urea and fatty acid metabolism. Adrenergic hepatic function in adults is mainly under the control of alpha(1)-ARs; however, the mechanism through which they influence diverse processes remains incompletely understood. This study describes a novel alpha(1)-AR-mediated transactivation of signal transducer and activator of transcription-3 (Stat3) in primary and transformed hepatocytes. Treatment of primary rat hepatocytes with the alpha(1)-AR agonist, phenylephrine (PE), induced a rapid phosphorylation of Stat3. PE also increased Stat3 phosphorylation, DNA binding and transcription activity in transformed human hepatocellular carcinoma cells (Hep3B). The PE-induced Stat3 phosphorylation, DNA binding and reporter activity were completely blocked by the selective alpha(1)-AR antagonist, prazosin. In addition, transfection of Hep3B cells with human alpha(1B)-AR expression vector also enhanced Stat3 phosphorylation and reporter activity. Moreover, overexpression of RGS2, a protein inhibitor of G(q/11) signaling, blocked PE-induced Stat3 phosphorylation and reporter activity. The observations that PE induced the formation of c-Src-Stat3 binding complex and phosphorylation of epidermal growth factor receptor (EGFR) and that inhibiting Src and EGFR prevented PE-induced Stat3 activation indicate the involvement of Src and EGFR. Taken together, these observations demonstrate a novel alpha(1)-AR-mediated Stat3 activation that involves G(q/11), Src, and EGFR in hepatic cells.
(c) 2008 Wiley-Liss, Inc.
Figures




Similar articles
-
Transactivated EGFR mediates α₁-AR-induced STAT3 activation and cardiac hypertrophy.Am J Physiol Heart Circ Physiol. 2011 Nov;301(5):H1941-51. doi: 10.1152/ajpheart.00338.2011. Epub 2011 Aug 19. Am J Physiol Heart Circ Physiol. 2011. PMID: 21856923
-
15-Lipoxygenase-1-enhanced Src-Janus kinase 2-signal transducer and activator of transcription 3 stimulation and monocyte chemoattractant protein-1 expression require redox-sensitive activation of epidermal growth factor receptor in vascular wall remodeling.J Biol Chem. 2011 Jun 24;286(25):22478-88. doi: 10.1074/jbc.M111.225060. Epub 2011 May 2. J Biol Chem. 2011. PMID: 21536676 Free PMC article.
-
Cross-talk between alpha(1B)-adrenergic receptor (alpha(1B)AR) and interleukin-6 (IL-6) signaling pathways. Activation of alpha(1b)AR inhibits il-6-activated STAT3 in hepatic cells by a p42/44 mitogen-activated protein kinase-dependent mechanism.J Biol Chem. 1999 Dec 10;274(50):35492-8. doi: 10.1074/jbc.274.50.35492. J Biol Chem. 1999. PMID: 10585421
-
Constitutively active Galpha16 stimulates STAT3 via a c-Src/JAK- and ERK-dependent mechanism.J Biol Chem. 2003 Dec 26;278(52):52154-65. doi: 10.1074/jbc.M307299200. Epub 2003 Oct 9. J Biol Chem. 2003. PMID: 14551213
-
α(1A)-adrenergic receptor differentially regulates STAT3 phosphorylation through PKCϵ and PKCδ in myocytes.J Recept Signal Transduct Res. 2012 Apr;32(2):76-86. doi: 10.3109/10799893.2011.647353. Epub 2012 Jan 24. J Recept Signal Transduct Res. 2012. PMID: 22268811 Free PMC article.
Cited by
-
GM-CSF protects rat photoreceptors from death by activating the SRC-dependent signalling and elevating anti-apoptotic factors and neurotrophins.Graefes Arch Clin Exp Ophthalmol. 2012 May;250(5):699-712. doi: 10.1007/s00417-012-1932-9. Graefes Arch Clin Exp Ophthalmol. 2012. PMID: 22297538
-
Sympathetic nervous system in tumor progression and metabolic regulation: mechanisms and clinical potential.J Transl Med. 2025 Jul 25;23(1):836. doi: 10.1186/s12967-025-06657-2. J Transl Med. 2025. PMID: 40713825 Free PMC article. Review.
-
Liver regeneration: biological and pathological mechanisms and implications.Nat Rev Gastroenterol Hepatol. 2021 Jan;18(1):40-55. doi: 10.1038/s41575-020-0342-4. Epub 2020 Aug 6. Nat Rev Gastroenterol Hepatol. 2021. PMID: 32764740 Review.
-
Constitutive SRC-mediated phosphorylation of pannexin 1 at tyrosine 198 occurs at the plasma membrane.J Biol Chem. 2019 Apr 26;294(17):6940-6956. doi: 10.1074/jbc.RA118.006982. Epub 2019 Feb 27. J Biol Chem. 2019. PMID: 30814251 Free PMC article.
-
Pathophysiologic Role of Neurotransmitters in Digestive Diseases.Front Physiol. 2021 Jun 14;12:567650. doi: 10.3389/fphys.2021.567650. eCollection 2021. Front Physiol. 2021. PMID: 34194334 Free PMC article. Review.
References
-
- Bertelsen LS, Barrett KE, Keely SJ. Gs protein-coupled receptor agonists induce transactivation of the epidermal growth factor receptor in T84 cells: implications for epithelial secretory responses. J Biol Chem. 2004;279(8):6271–6279. - PubMed
-
- Boccaccio C, Ando M, Tamagnone L, Bardelli A, Michieli P, Battistini C, Comoglio PM. Induction of epithelial tubules by growth factor HGF depends on the STAT pathway. Nature. 1998;391(6664):285–288. - PubMed
-
- Boluyt MO, Zheng JS, Younes A, Long X, O’Neill L, Silverman H, Lakatta EG, Crow MT. Rapamycin inhibits alpha 1-adrenergic receptor-stimulated cardiac myocyte hypertrophy but not activation of hypertrophy-associated genes. Evidence for involvement of p70 S6 kinase. Circ Res. 1997;81(2):176–186. - PubMed
-
- Bromberg JF, Wrzeszczynska MH, Devgan G, Zhao Y, Pestell RG, Albanese C, Darnell JE., Jr Stat3 as an oncogene. Cell. 1999;98(3):295–303. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

