Spectroscopic and density functional theory studies of the blue-copper site in M121SeM and C112SeC azurin: Cu-Se versus Cu-S bonding
- PMID: 18314977
- PMCID: PMC2713798
- DOI: 10.1021/ja076495a
Spectroscopic and density functional theory studies of the blue-copper site in M121SeM and C112SeC azurin: Cu-Se versus Cu-S bonding
Abstract
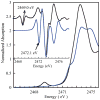
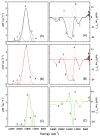
S K-edge X-ray absorption, UV-vis absorption, magnetic circular dichroism (MCD), and resonance Raman spectroscopies are used to investigate the electronic structure differences among WT, M121SeM, and C112SeC Pseudomonas aeruginosa (P.a) azurin. A comparison of S K-edge XAS of WT and M121SeM azurin and a CuII-thioether model complex shows that the 38% S character in the ground state wave function of the blue-copper (BC) sites solely reflects the Cu-SCys bond. Resonance Raman (rR) data on WT and C112SeC azurin give direct evidence for the kinematic coupling between the Cu-SCys stretch and the cysteine deformation modes in WT azurin, which leads to multiple features in the rR spectrum of the BC site. The UV-vis absorption and MCD data on WT, M121SeM, and C112SeC give very similar C0/D0 ratios, indicating that the C-term MCD intensity mechanism involves Cu-centered spin-orbit coupling (SOC). The spectroscopic data combined with density functional theory (DFT) calculations indicate that SCys and SeCys have similar covalent interactions with Cu at their respective bond lengths of 2.1 and 2.3 A. This reflects the similar electronegativites of S and Se in the thiolate/selenolate ligand fragment and explains the strong spectroscopic similarities between WT and C112SeC azurin.
Figures






Similar articles
-
Resonance Raman spectroscopy of the azurin His117Gly mutant. Interconversion of type 1 and type 2 copper sites through exogenous ligands.Biochemistry. 1993 Nov 23;32(46):12455-64. doi: 10.1021/bi00097a025. Biochemistry. 1993. PMID: 8241136
-
Spectroscopic and DFT studies of second-sphere variants of the type 1 copper site in azurin: covalent and nonlocal electrostatic contributions to reduction potentials.J Am Chem Soc. 2012 Oct 10;134(40):16701-16. doi: 10.1021/ja306438n. Epub 2012 Oct 2. J Am Chem Soc. 2012. PMID: 22985400 Free PMC article.
-
Spectroscopic characterizations of bridging cysteine ligand variants of an engineered Cu2(Scys)2 CuA azurin.Inorg Chem. 2006 Jan 9;45(1):102-7. doi: 10.1021/ic051375u. Inorg Chem. 2006. PMID: 16390045
-
Cu(A) centers and their biosynthetic models in azurin.J Biol Inorg Chem. 2010 May;15(4):461-83. doi: 10.1007/s00775-010-0625-2. Epub 2010 Feb 19. J Biol Inorg Chem. 2010. PMID: 20169379 Review.
-
Rack-induced bonding in blue-copper proteins.Eur J Biochem. 1994 Aug 1;223(3):711-8. doi: 10.1111/j.1432-1033.1994.tb19044.x. Eur J Biochem. 1994. PMID: 8055947 Review.
Cited by
-
Inner- and outer-sphere metal coordination in blue copper proteins.J Inorg Biochem. 2012 Oct;115:119-26. doi: 10.1016/j.jinorgbio.2012.05.002. Epub 2012 May 9. J Inorg Biochem. 2012. PMID: 22658756 Free PMC article. Review.
-
Crucial role of copper in detection of metal-coordinating odorants.Proc Natl Acad Sci U S A. 2012 Feb 28;109(9):3492-7. doi: 10.1073/pnas.1111297109. Epub 2012 Feb 10. Proc Natl Acad Sci U S A. 2012. PMID: 22328155 Free PMC article.
-
The essential role of the Cu(II) state of Sco in the maturation of the Cu(A) center of cytochrome oxidase: evidence from H135Met and H135SeM variants of the Bacillus subtilis Sco.J Biol Inorg Chem. 2011 Feb;16(2):285-97. doi: 10.1007/s00775-010-0725-z. Epub 2010 Oct 31. J Biol Inorg Chem. 2011. PMID: 21069401 Free PMC article.
-
Design of Heteronuclear Metalloenzymes.Methods Enzymol. 2016;580:501-37. doi: 10.1016/bs.mie.2016.05.050. Epub 2016 Jul 26. Methods Enzymol. 2016. PMID: 27586347 Free PMC article.
-
Converged Structural and Spectroscopic Properties for Refined QM/MM Models of Azurin.Inorg Chem. 2021 May 17;60(10):7399-7412. doi: 10.1021/acs.inorgchem.1c00640. Epub 2021 May 3. Inorg Chem. 2021. PMID: 33939922 Free PMC article.
References
-
- Sykes AG. Adv Inorg Chem. 1991;36:377–408.
-
- Gray HB, Malmström BG, Williams RJP. J Biol Inorg Chem. 2000;5:551–559. - PubMed
-
- Messerschmidt A. Struct Bonding. 1998;90:37–68.
-
- Solomon EI, Szilagyi RK, DeBeer George S, Basumallick L. Chem Rev. 2004;104:419–458. - PubMed
-
- Solomon EI, Penfield KW, Gewirth AA, Lowery MD, Shadle SE, Guckert JA, LaCroix LB. Inorg Chim Acta. 1996;243:67–78.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

