The evolutionary costs of immunological maintenance and deployment
- PMID: 18315877
- PMCID: PMC2292698
- DOI: 10.1186/1471-2148-8-76
The evolutionary costs of immunological maintenance and deployment
Abstract
Background: The evolution of disease resistance and immune function may be limited if increased immunocompetence comes at the expense of other fitness-determining traits. Both the maintenance of an immune system and the deployment of an immune response can be costly, and the observed costs may be evaluated as either physiological or evolutionary in origin. Evolutionary costs of immunological maintenance are revealed as negative genetic correlations between immunocompetence and fitness in the absence of infection. Costs of deployment are most often studied as physiological costs associated with immune system induction, however, evolutionary costs of deployment may also be present if genotypes vary in the extent of the physiological cost experienced.
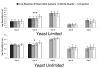
Results: In this study we analyzed evolutionary and physiological costs of immunity in two environments representing food-limited and food-unlimited conditions. Patterns of genetic variation were estimated in females from 40 'hemiclone families' isolated from a population of D. melanogaster. Phenotypes evaluated included fecundity, weight measures at different time periods and resistance to Providencia rettgeri, a naturally occurring Gram-negative pathogen of D. melanogaster. In the food-limited environment we found a negative genetic correlation between fecundity in the absence of infection and resistance, indicative of an evolutionary cost of maintenance. No such correlation was observed in the food-unlimited environment, and the slopes of these correlations significantly differed, demonstrating a genotype-by-environment interaction for the cost of maintenance. Physiological costs of deployment were also observed, but costs were primarily due to wounding. Deployment costs were slightly exaggerated in the food-limited environment. Evolutionary costs of immunological deployment on fecundity were not observed, and there was only marginally significant genetic variation in the cost expressed by changes in dry weight.
Conclusion: Our results suggest that the costs of immunity may be an important factor limiting the evolution of resistance in food-limited environments. However, the significant genotype-by-environment interaction for maintenance costs, combined with the observation that deployment costs were partially mitigated in the food-unlimited environment, emphasizes the importance of considering environmental variation when estimating patterns of genetic variance and covariance, and the dubious nature of predicting evolutionary responses to selection from quantitative genetic estimates carried out in a single environment.
Figures


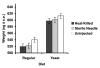


Similar articles
-
Genotype and diet shape resistance and tolerance across distinct phases of bacterial infection.BMC Evol Biol. 2014 Mar 22;14(1):56. doi: 10.1186/1471-2148-14-56. BMC Evol Biol. 2014. PMID: 24655914 Free PMC article.
-
Genotype-by-environment interactions and adaptation to local temperature affect immunity and fecundity in Drosophila melanogaster.PLoS Pathog. 2008 Mar 14;4(3):e1000025. doi: 10.1371/journal.ppat.1000025. PLoS Pathog. 2008. PMID: 18369474 Free PMC article.
-
The complex contributions of genetics and nutrition to immunity in Drosophila melanogaster.PLoS Genet. 2015 Mar 12;11(3):e1005030. doi: 10.1371/journal.pgen.1005030. eCollection 2015 Mar. PLoS Genet. 2015. PMID: 25764027 Free PMC article.
-
Costs of resistance in insect-parasite and insect-parasitoid interactions.Parasitology. 2002;125 Suppl:S71-82. doi: 10.1017/s0031182002001750. Parasitology. 2002. PMID: 12622330 Review.
-
Evolution of host resistance and parasitoid counter-resistance.Adv Parasitol. 2009;70:257-80. doi: 10.1016/S0065-308X(09)70010-7. Adv Parasitol. 2009. PMID: 19773074 Review.
Cited by
-
Effects of immune challenge on expression of life-history and immune trait expression in sexually reproducing metazoans-a meta-analysis.BMC Biol. 2020 Oct 7;18(1):135. doi: 10.1186/s12915-020-00856-7. BMC Biol. 2020. PMID: 33028304 Free PMC article.
-
Intraspecific genetic variation in host vigour, viral load and disease tolerance during Drosophila C virus infection.Open Biol. 2023 Mar;13(3):230025. doi: 10.1098/rsob.230025. Epub 2023 Mar 1. Open Biol. 2023. PMID: 36854375 Free PMC article.
-
No effect of mitochondrial genotype on reproductive plasticity following exposure to a non-infectious pathogen challenge in female or male Drosophila.Sci Rep. 2017 Feb 9;7:42009. doi: 10.1038/srep42009. Sci Rep. 2017. PMID: 28181526 Free PMC article.
-
Parallel and costly changes to cellular immunity underlie the evolution of parasitoid resistance in three Drosophila species.PLoS Pathog. 2017 Oct 19;13(10):e1006683. doi: 10.1371/journal.ppat.1006683. eCollection 2017 Oct. PLoS Pathog. 2017. PMID: 29049362 Free PMC article.
-
Sex, offspring and carcass determine antimicrobial peptide expression in the burying beetle.Sci Rep. 2016 May 3;6:25409. doi: 10.1038/srep25409. Sci Rep. 2016. PMID: 27139635 Free PMC article.
References
-
- Anderson RM, May RM. Coevolution of hosts and parasites. Parasitology. 1982;85(2):411–426. - PubMed
-
- Grenfell BT, Dobson AP. Ecology of infectious diseases in natural populations. Cambridge: Cambridge University Press; 1995.
-
- Kraaijeveld AR, Godfray HCJ. Trade-off between parasitoid resistance and larval competitive ability in Drosophila melanogaster. Nature. 1997;389(6648):278–280. - PubMed
-
- Lazzaro BP, Sceurman BK, Clark AG. Genetic basis of natural variation in D. melanogaster antibacterial immunity. Science. 2004;303(5665):1873–1876. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

