Absence seizures in C3H/HeJ and knockout mice caused by mutation of the AMPA receptor subunit Gria4
- PMID: 18316356
- PMCID: PMC2405903
- DOI: 10.1093/hmg/ddn064
Absence seizures in C3H/HeJ and knockout mice caused by mutation of the AMPA receptor subunit Gria4
Abstract
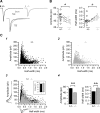
Absence epilepsy, characterized by spike-wave discharges (SWD) in the electroencephalogram, arises from aberrations within the circuitry of the cerebral cortex and thalamus that regulates awareness. The inbred mouse strain C3H/HeJ is prone to absence seizures, with a major susceptibility locus, spkw1, accounting for most of the phenotype. Here we find that spkw1 is associated with a hypomorphic retroviral-like insertion mutation in the Gria4 gene, encoding one of the four amino-3-hydroxy-5-methyl-4isoxazolepropionic acid (AMPA) receptor subunits in the brain. Consistent with this, Gria4 knockout mice also have frequent SWD and do not complement spkw1. In contrast, null mutants for the related gene Gria3 do not have SWD, and Gria3 loss actually lowers SWD of spkw1 homozygotes. Gria3 and Gria4 encode the predominant AMPA receptor subunits in the reticular thalamus, which is thought to play a central role in seizure genesis by inhibiting thalamic relay cells and promoting rebound burst firing responses. In Gria4 mutants, synaptic excitation of inhibitory reticular thalamic neurons is enhanced, with increased duration of synaptic responses-consistent with what might be expected from reduction of the kinetically faster subunit of AMPA receptors encoded by Gria4. These results demonstrate for the first time an essential role for Gria4 in the brain, and suggest that abnormal AMPA receptor-dependent synaptic activity can be involved in the network hypersynchrony that underlies absence seizures.
Figures
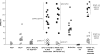



References
-
- Wyllie E. The Treatment of Epilepsy: Principles and Practice. Philadelphia: Lea & Febiger; 1993.
-
- McCormick D.A., Contreras D. On the cellular and network bases of epileptic seizures. Annu. Rev. Physiol. 2001;63:815–846. - PubMed
-
- Avanzini G., Vergnes M., Spreafico R., Marescaux C. Calcium-dependent regulation of genetically determined spike and waves by the reticular thalamic nucleus of rats. Epilepsia. 1993;34:1–7. - PubMed
-
- Berkovic S.F., Howell R.A., Hay D.A., Hopper J.L. Epilepsies in twins: genetics of the major epilepsy syndromes. Ann. Neurol. 1998;43:435–445. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

