A microsphere-based vaccine prevents and reverses new-onset autoimmune diabetes
- PMID: 18316361
- PMCID: PMC2713034
- DOI: 10.2337/db07-0507
A microsphere-based vaccine prevents and reverses new-onset autoimmune diabetes
Abstract
Objective: This study was aimed at ascertaining the efficacy of antisense oligonucleotide-formulated microspheres to prevent type 1 diabetes and to reverse new-onset disease.
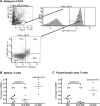
Research design and methods: Microspheres carrying antisense oligonucleotides to CD40, CD80, and CD86 were delivered into NOD mice. Glycemia was monitored to determine disease prevention and reversal. In recipients that remained and/or became diabetes free, spleen and lymph node T-cells were enriched to determine the prevalence of Foxp3(+) putative regulatory T-cells (Treg cells). Splenocytes from diabetes-free microsphere-treated recipients were adoptively cotransferred with splenocytes from diabetic NOD mice into NOD-scid recipients. Live-animal in vivo imaging measured the microsphere accumulation pattern. To rule out nonspecific systemic immunosuppression, splenocytes from successfully treated recipients were pulsed with beta-cell antigen or ovalbumin or cocultured with allogeneic splenocytes.
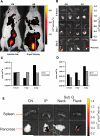
Results: The microspheres prevented type 1 diabetes and, most importantly, exhibited a capacity to reverse clinical hyperglycemia, suggesting reversal of new-onset disease. The microspheres augmented Foxp3(+) Treg cells and induced hyporesponsiveness to NOD-derived pancreatic beta-cell antigen, without compromising global immune responses to alloantigens and nominal antigens. T-cells from successfully treated mice suppressed adoptive transfer of disease by diabetogenic splenocytes into secondary immunodeficient recipients. Finally, microspheres accumulated within the pancreas and the spleen after either intraperitoneal or subcutaneous injection. Dendritic cells from spleen of the microsphere-treated mice exhibit decreased cell surface CD40, CD80, and CD86.
Conclusions: This novel microsphere formulation represents the first diabetes-suppressive and reversing nucleic acid vaccine that confers an immunoregulatory phenotype to endogenous dendritic cells.
Figures





References
-
- Atkinson MA, Eisenbarth GS. Type 1 diabetes: new perspectives on disease pathogenesis and treatment. Lancet. 2001;358:221–229. - PubMed
-
- Thomas HE, Darwiche R, Corbett JA, Kay TW. Interleukin-1 plus gamma-interferon-induced pancreatic β-cell dysfunction is mediated by β-cell nitric oxide production. Diabetes. 2002;51:311–316. - PubMed
-
- Abdul-Rasoul M, Habib H, Al-Khouly M. “The honeymoon phase ” in children with type 1 diabetes mellitus: frequency, duration, and influential factors. Pediatr Diabetes. 2006;7:101–107. - PubMed
-
- Bougneres PF, Landais P, Boisson C, Carel JC, Frament N, Boitard C, Chaussain JL, Bach JF. Limited duration of remission of insulin dependency in children with recent overt type I diabetes treated with low-dose cyclosporin. Diabetes. 1990;39:1264–1272. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

