Functional analysis of effector and regulatory T cells in a parasitic nematode infection
- PMID: 18316386
- PMCID: PMC2346705
- DOI: 10.1128/IAI.01233-07
Functional analysis of effector and regulatory T cells in a parasitic nematode infection
Abstract
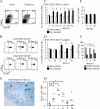
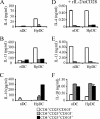
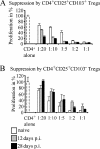
Parasitic nematodes typically modulate T-cell reactivity, primarily during the chronic phase of infection. We analyzed the role of CD4-positive (CD4+) T effector (T(eff)) cells and regulatory T (T(reg)) cells derived from mice chronically infected with the intestinal nematode Heligmosomoides polygyrus. Different CD4+ T-cell subsets were transferred into naïve recipients that were subsequently infected with H. polygyrus. Adoptive transfer of conventional T(eff) cells conferred protection and led to a significant decrease in the worm burdens of H. polygyrus-infected recipients. Roughly 0.2% of the CD4+ T cells were H. polygyrus specific based on expression of CD154, and cells producing interleukin 4 (IL-4) and IL-13 were highly enriched within the CD154+ population. In contrast, adoptive transfer of T(reg) cells, characterized by the markers CD25 and CD103 and the transcription factor Foxp3, had no effect on the worm burdens of recipients. Further analysis showed that soon after infection, the number of Foxp3+ T(reg) cells temporarily increased in the inflamed tissue while effector/memory-like CD103+ Foxp+ T(reg) cells systemically increased in the draining lymph nodes and spleen. In addition, T(reg) cells represented a potential source of IL-10 and reduced the expression of IL-4. Finally, under in vitro conditions, T(reg) cells from infected mice were more potent suppressors than cells derived from naïve mice. In conclusion, our data indicate that small numbers of T(eff) cells have the ability to promote host protective immune responses, even in the presence of T(reg) cells.
Figures
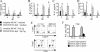


References
-
- Akiho, H., Y. Deng, P. Blennerhassett, H. Kanbayashi, and S. M. Collins. 2005. Mechanisms underlying the maintenance of muscle hypercontractility in a model of postinfective gut dysfunction. Gastroenterology 129131-141. - PubMed
-
- Al-Qaoud, K. M., B. Fleischer, and A. Hoerauf. 1998. The Xid defect imparts susceptibility to experimental murine filariosis—association with a lack of antibody and IL-10 production by B cells in response to phosphorylcholine. Int. Immunol. 1017-25. - PubMed
-
- Babu, S., C. P. Blauvelt, V. Kumaraswami, and T. B. Nutman. 2006. Regulatory networks induced by live parasites impair both TH1 and TH2 pathways in patent lymphatic filariasis: implications for parasite persistence. J. Immunol. 1763248-3256. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

