Pseudomonas aeruginosa induces membrane blebs in epithelial cells, which are utilized as a niche for intracellular replication and motility
- PMID: 18316391
- PMCID: PMC2346716
- DOI: 10.1128/IAI.01221-07
Pseudomonas aeruginosa induces membrane blebs in epithelial cells, which are utilized as a niche for intracellular replication and motility
Abstract
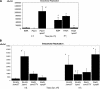
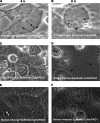
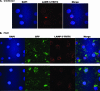
Pseudomonas aeruginosa is known to invade epithelial cells during infection and in vitro. However, little is known of bacterial or epithelial factors modulating P. aeruginosa intracellular survival or replication after invasion, except that it requires a complete lipopolysaccharide core. In this study, real-time video microscopy revealed that invasive P. aeruginosa isolates induced the formation of membrane blebs in multiple epithelial cell types and that these were then exploited for intracellular replication and rapid real-time motility. Further studies revealed that the type three secretion system (T3SS) of P. aeruginosa was required for blebbing. Mutants lacking either the entire T3SS or specific T3SS components were instead localized to intracellular perinuclear vacuoles. Most T3SS mutants that trafficked to perinuclear vacuoles gradually lost intracellular viability, and vacuoles containing those bacteria were labeled by the late endosomal marker lysosome-associated marker protein 3 (LAMP-3). Interestingly, mutants deficient only in the T3SS translocon structure survived and replicated within the vacuoles that did not label with LAMP-3. Taken together, these data suggest two novel roles of the P. aeruginosa T3SS in enabling bacterial intracellular survival: translocon-dependent formation of membrane blebs, which form a host cell niche for bacterial growth and motility, and effector-dependent bacterial survival and replication within intracellular perinuclear vacuoles.
Figures



References
-
- Aktories, K., and J. T. Barbieri. 2005. Bacterial cytotoxins: targeting eukaryotic switches. Nat. Rev. 3397-410. - PubMed
-
- Alonso, A., and F. Garcia-del Portillo. 2004. Hijacking of eukaryotic functions by intracellular bacterial pathogens. Int. Microbiol. 7181-191. - PubMed
-
- Amano, A., I. Nakagawa, and T. Yoshimori. 2006. Autophagy in innate immunity against intracellular bacteria. J. Biochem. (Tokyo) 140161-166. - PubMed
-
- Baca, O. G., Y. P. Li, and H. Kumar. 1994. Survival of the Q fever agent Coxiella burnetii in the phagolysosome. Trends Microbiol. 2476-480. - PubMed
-
- Barbieri, J. T., and J. Sun. 2004. Pseudomonas aeruginosa ExoS and ExoT. Rev. Physiol. Biochem. Pharmacol. 15279-92. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

