LRP-1 silencing prevents malignant cell invasion despite increased pericellular proteolytic activities
- PMID: 18316405
- PMCID: PMC2293087
- DOI: 10.1128/MCB.02238-07
LRP-1 silencing prevents malignant cell invasion despite increased pericellular proteolytic activities
Abstract
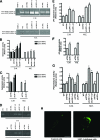
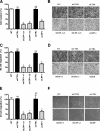
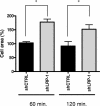
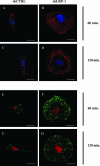
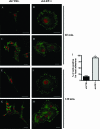
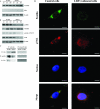
The scavenger receptor low-density lipoprotein receptor-related protein 1 (LRP-1) mediates the clearance of a variety of biological molecules from the pericellular environment, including proteinases which degrade the extracellular matrix in cancer progression. However, its accurate functions remain poorly explored and highly controversial. Here we show that LRP-1 silencing by RNA interference results in a drastic inhibition of cell invasion despite a strong stimulation of pericellular matrix metalloproteinase 2 and urokinase-type plasminogen activator proteolytic activities. Cell migration in both two and three dimensions is decreased by LRP-1 silencing. LRP-1-silenced carcinoma cells, which are characterized by major cytoskeleton rearrangements, display atypical overspread morphology with a lack of membrane extensions. LRP-1 silencing accelerates cell attachment, inhibits cell-substrate deadhesion, and induces the accumulation, at the cell periphery, of abundant talin-containing focal adhesion complexes deprived of FAK and paxillin. We conclude that in addition to its role in ligand binding and endocytosis, LRP-1 regulates cytoskeletal organization and adhesive complex turnover in malignant cells by modulating the focal complex composition, thereby promoting invasion.
Figures




References
-
- Barmina, O. Y., H. W. Walling, G. J. Fiacco, J. M. Freije, C. Lopez-Otin, J. J. Jeffrey, and N. C. Partridge. 1999. Collagenase-3 binds to a specific receptor and requires the low density lipoprotein receptor-related protein for internalization. J. Biol. Chem. 27430087-30093. - PubMed
-
- Boucher, P., M. Gotthardt, W. P. Li, R. G. Anderson, and J. Herz. 2003. LRP: role in vascular wall integrity and protection from atherosclerosis. Science 300329-332. - PubMed
-
- Bu, G., P. A. Morton, and A. L. Schwartz. 1992. Identification and partial characterization by chemical cross-linking of a binding protein for tissue-type plasminogen activator (t-PA) on rat hepatoma cells. A plasminogen activator inhibitor type 1-independent t-PA receptor. J. Biol. Chem. 26715595-15602. - PubMed
-
- Chazaud, B., S. Bonavaud, A. Plonquet, M. Pouchelet, R. K. Gherardi, and G. Barlovatz-Meimon. 2000. Involvement of the [uPAR:uPA:PAI-1:LRP] complex in human myogenic cell motility. Exp. Cell Res. 258237-244. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
