Allergic pulmonary inflammation in mice is dependent on eosinophil-induced recruitment of effector T cells
- PMID: 18316417
- PMCID: PMC2275390
- DOI: 10.1084/jem.20071840
Allergic pulmonary inflammation in mice is dependent on eosinophil-induced recruitment of effector T cells
Abstract
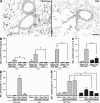
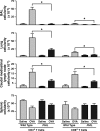
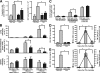
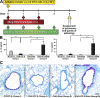
The current paradigm surrounding allergen-mediated T helper type 2 (Th2) immune responses in the lung suggests an almost hegemonic role for T cells. Our studies propose an alternative hypothesis implicating eosinophils in the regulation of pulmonary T cell responses. In particular, ovalbumin (OVA)-sensitized/challenged mice devoid of eosinophils (the transgenic line PHIL) have reduced airway levels of Th2 cytokines relative to the OVA-treated wild type that correlated with a reduced ability to recruit effector T cells to the lung. Adoptive transfer of Th2-polarized OVA-specific transgenic T cells (OT-II) alone into OVA-challenged PHIL recipient mice failed to restore Th2 cytokines, airway histopathologies, and, most importantly, the recruitment of pulmonary effector T cells. In contrast, the combined transfer of OT-II cells and eosinophils into PHIL mice resulted in the accumulation of effector T cells and a concomitant increase in both airway Th2 immune responses and histopathologies. Moreover, we show that eosinophils elicit the expression of the Th2 chemokines thymus- and activation-regulated chemokine/CCL17 and macrophage-derived chemokine/CCL22 in the lung after allergen challenge, and blockade of these chemokines inhibited the recruitment of effector T cells. In summary, the data suggest that pulmonary eosinophils are required for the localized recruitment of effector T cells.
Figures

References
-
- Larche, M., D.S. Robinson, and A.B. Kay. 2003. The role of T lymphocytes in the pathogenesis of asthma. J. Allergy Clin. Immunol. 111:450–463. - PubMed
-
- Gavett, S.H., X. Chen, F. Finkelman, and M. Wills-Karp. 1994. Depletion of murine CD4+ T lymphocytes prevents antigen-induced airway hyperreactivity and pulmonary eosinophilia. Am. J. Respir. Cell Mol. Biol. 10:587–593. - PubMed
-
- Bousquet, J., P. Chanez, J.Y. Lacoste, G. Barneon, N. Ghavanian, I. Enander, P. Venge, S. Ahlstedt, J. Simony-Lafontaine, P. Godard, and M. Francois-Bernard. 1990. Eosinophilic inflammation in asthma. N. Engl. J. Med. 323:1033–1039. - PubMed
-
- Humbles, A.A., C.M. Lloyd, S.J. McMillan, D.S. Friend, G. Xanthou, E.E. McKenna, S. Ghiran, N.P. Gerard, C. Yu, S.H. Orkin, and C. Gerard. 2004. A critical role for eosinophils in allergic airways remodeling. Science. 305:1776–1779. - PubMed
-
- Lee, J.J., D. Dimina, M.P. Macias, S.I. Ochkur, M.P. McGarry, K.R. O'Neill, C. Protheroe, R. Pero, T. Nguyen, S.A. Cormier, et al. 2004. Defining a link with asthma in mice congenitally deficient in eosinophils. Science. 305:1773–1776. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

