Allogeneic granulocyte macrophage colony-stimulating factor-secreting tumor immunotherapy alone or in sequence with cyclophosphamide for metastatic pancreatic cancer: a pilot study of safety, feasibility, and immune activation
- PMID: 18316569
- PMCID: PMC2879140
- DOI: 10.1158/1078-0432.CCR-07-0371
Allogeneic granulocyte macrophage colony-stimulating factor-secreting tumor immunotherapy alone or in sequence with cyclophosphamide for metastatic pancreatic cancer: a pilot study of safety, feasibility, and immune activation
Abstract
Purpose: The combination of chemotherapy and immunotherapy has not been examined in patients with advanced pancreatic cancer. We conducted a study of two granulocyte macrophage colony-stimulating factor-secreting pancreatic cancer cell lines (CG8020/CG2505) as immunotherapy administered alone or in sequence with cyclophosphamide in patients with advanced pancreatic cancer.
Experimental design: This was an open-label study with two cohorts: cohort A, 30 patients administered a maximum of six doses of CG8020/CG2505 at 21-day intervals; and cohort B, 20 patients administered 250 mg/m(2) of cyclophosphamide i.v. 1 day before the same immunotherapy given as in cohort A. The primary objective was to evaluate safety and duration of immunity. Secondary objectives included time to disease progression and median overall survival.
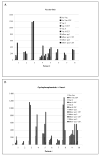
Results: The administration of CG8020/CG2505 alone or in sequence with cyclophosphamide showed minimal treatment-related toxicity. Median survival values in cohort A and cohort B were 2.3 and 4.3 months, respectively. CD8(+) T-cell responses to HLA class I-restricted mesothelin epitopes were identified predominantly in patients treated with cyclophosphamide + CG8020/CG2505 immunotherapy.
Conclusion: Granulocyte macrophage colony-stimulating factor-secreting pancreatic cancer cell lines CG8020/CG2505 alone or in sequence with cyclophosphamide showed minimal treatment-related toxicity in patients with advanced pancreatic cancer. Also, mesothelin-specific T-cell responses were detected/enhanced in some patients treated with CG8020/CG2505 immunotherapy. In addition, cyclophosphamide-modulated immunotherapy resulted in median survival in a gemcitabine-resistant population similar to chemotherapy alone. These findings support additional investigation of cyclophosphamide with CG8020/CG2505 immunotherapy in patients with advanced pancreatic cancer.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures
References
-
- Jemal A, Siegel R, Ward E, et al. Cancer Statistics 2006. CA Cancer J Clin. 2006;56:106–130. - PubMed
-
- Yeo CJ, Pluth-Yeo T, Hruban R, et al. Cancer of the Pancreas. In: DeVita VT, editor. Principles and Practice of Oncology. Seventh. Philadelphia: J.B. Lippincott Co; 2005. pp. 945–986.
-
- Burris HA, Moore MJ, Cripps MC, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreatic cancer: A randomized trial. J Clin Oncol. 1997;15:2403–13. - PubMed
-
- Heinemann V, Quietzsch D, Gieseler F, et al. Randomized phase III trial of gemcitabine plus cisplatin compared with gemcitabine alone in advanced pancreatic cancer. J Clin Oncol. 2006;24:3946–52. - PubMed
-
- Rocha Lima CM, Green MR, Rotche R, et al. Irinotecan plus gemcitabine results in no survival advantage compared with gemcitabine monotherapy in patients with locally advanced or metastatic pancreatic cancer despite increased tumor response rate. J Clin Oncol. 2004;22:3776–83. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

