Cloning and identification of an oxytocin/vasopressin-like receptor and its ligand from insects
- PMID: 18316733
- PMCID: PMC2265169
- DOI: 10.1073/pnas.0710897105
Cloning and identification of an oxytocin/vasopressin-like receptor and its ligand from insects
Abstract
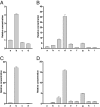
More than 20 years ago, an oxytocin/vasopressin-like peptide, CLITNCPRGamide, was isolated from the locust, Locusta migratoria [Proux JP, et al. (1987) Identification of an arginine vasopressin-like diuretic hormone from Locusta migratoria. Biochem Biophys Res Commun 149:180-186]. However, no similar peptide could be identified in other insects, nor could its prohormone be cloned, or its physiological actions be established. Here, we report that the recently sequenced genome from the red flour beetle Tribolium castaneum contains a gene coding for an oxytocin/vasopressin-like peptide, identical to the locust peptide, which we named inotocin (for insect oxytocin/vasopressin-like peptide) and a gene coding for an inotocin G protein-coupled receptor (GPCR). We cloned the Tribolium inotocin preprohormone and the inotocin GPCR and expressed the GPCR in CHO cells. This GPCR is strongly activated by low concentrations of inotocin (EC(50), 5 x 10(-9) M), demonstrating that it is the inotocin receptor. Quantitative RT-PCR (qPCR) showed that in adult Tribolium, the receptor is mainly expressed in the head and much less in the hindgut and Malpighian tubules, suggesting that the inotocin/receptor couple does not play a role in water homeostasis. Surprisingly, qPCR also showed that the receptor is 30x more expressed in the first larval stages than in adult animals. The inotocin/receptor couple can also be found in the recently sequenced genome from the parasitic wasp Nasonia vitripennis but not in any other holometabolous insect with a completely sequenced genome (12 Drosophila species, the malaria mosquito Anopheles gambiae, the yellow fever mosquito Aedes aegypti, the silk worm Bombyx mori, and the honey bee Apis mellifera), suggesting that this neuropeptide system is confined to basal holometabolous insects. Furthermore, we identified an oxytocin/vasopressin-like peptide and receptor in the recently sequenced genome from the water flea Daphnia pulex (Crustacea). To our knowledge, this is the first report on the molecular cloning of an oxytocin/vasopressin-like receptor and its ligand from arthropods.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
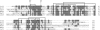

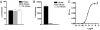

References
-
- Acher R, Chauvet J. La structure de la vasopressin de boeuf. Biochim Biophys Acta. 1953;12:487–488. - PubMed
-
- du Vigneaud V, Ressler C, Trippett S. The sequence of amino acids in oxytocin, with a proposal for the structure of oxytocin. J Biol Chem. 1953;205:949–957. - PubMed
-
- du Vigneaud V, Lawler HC, Popenoe EA. Enzymatic cleavage of glycinamide from vasopressin and a proposed structure for this pressor-antidiuretic hormone of the posterior pituitary. J Am Chem Soc. 1953;75:4880–4881.
-
- Ivell R, Schmale H, Richter D. Vasopressin and oxytocin precusors as model preprohormones. Neuroendocrinology. 1983;37:235–240. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources

