In vivo correction of a Menkes disease model using antisense oligonucleotides
- PMID: 18316734
- PMCID: PMC2268804
- DOI: 10.1073/pnas.0710865105
In vivo correction of a Menkes disease model using antisense oligonucleotides
Abstract
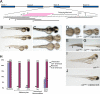
Although the molecular basis of many inherited metabolic diseases has been defined, the availability of effective therapies in such disorders remains problematic. Menkes disease is a fatal neurodegenerative disorder due to loss-of-function mutations in the ATP7A gene encoding a copper-transporting P-type Atpase. To develop therapeutic approaches in affected patients, we have identified a zebrafish model of Menkes disease termed calamity that results from splicing defects in the zebrafish orthologue of the ATP7A gene. Embryonic-recessive lethal mutants have impaired copper homeostasis that results in absent melanin pigmentation, impaired notochord formation, and hindbrain neurodegeneration. In this current study, we have attempted to rescue these striking phenotypic alterations by using a series of antisense morpholino oligonucleotides directed against the splice-site junctions of two mutant calamity alleles. Our findings reveal a robust and complete correction of the copper-deficient defects of calamity in association with the generation of the WT Menkes protein in all rescued mutants. Interestingly, a quantitative analysis of atp7a-specific transcripts suggests that competitive translational regulation may account for the synthesis of WT protein in these embryos. This in vivo correction of Menkes disease through the rescue of aberrant splicing may provide therapeutic options in this fatal disease and illustrates the potential for zebrafish models of human genetic disease in the development of treatments based on the principles of interactions of synthetic oligonucleotide analogues with mRNA.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Lutsenko S, Petris MJ. Function and regulation of the mammalian copper-transporting ATPases: Insights from biochemical and cell biological approaches. J Membr Biol. 2003;191:1–12. - PubMed
-
- Lutsenko S, Barnes NL, Bartee MY, Dmitriev OY. Function and regulation of human copper-transporting ATPases. Physiol Rev. 2007;87:1011–1046. - PubMed
-
- Culotta VC, Gitlin JD. In: The Molecular and Metabolic Basis of Inherited Disease. Scriver CR, Beaudet AL, Sly WS, Valle D, editors. New York: McGraw–Hill; 2001. pp. 3105–3136.
-
- Kaler SG. Metabolic and molecular bases of Menkes disease and occipital horn syndrome. Pediatr Dev Pathol. 1998;1:85–98. - PubMed
-
- Sheela SR, Latha M, Liu P, Lem K, Kaler SG. Copper-replacement treatment for symptomatic Menkes disease: Ethical considerations. Clin Genet. 2005;68:278–283. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

