Disclosure of the mycobacterial outer membrane: cryo-electron tomography and vitreous sections reveal the lipid bilayer structure
- PMID: 18316738
- PMCID: PMC2268800
- DOI: 10.1073/pnas.0709530105
Disclosure of the mycobacterial outer membrane: cryo-electron tomography and vitreous sections reveal the lipid bilayer structure
Abstract
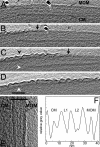
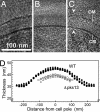
The cell walls of mycobacteria form an exceptional permeability barrier, and they are essential for virulence. They contain extractable lipids and long-chain mycolic acids that are covalently linked to peptidoglycan via an arabinogalactan network. The lipids were thought to form an asymmetrical bilayer of considerable thickness, but this could never be proven directly by microscopy or other means. Cryo-electron tomography of unperturbed or detergent-treated cells of Mycobacterium smegmatis embedded in vitreous ice now reveals the native organization of the cell envelope and its delineation into several distinct layers. The 3D data and the investigation of ultrathin frozen-hydrated cryosections of M. smegmatis, Myobacterium bovis bacillus Calmette-Guérin, and Corynebacterium glutamicum identified the outermost layer as a morphologically symmetrical lipid bilayer. The structure of the mycobacterial outer membrane necessitates considerable revision of the current view of its architecture. Conceivable models are proposed and discussed. These results are crucial for the investigation and understanding of transport processes across the mycobacterial cell wall, and they are of particular medical relevance in the case of pathogenic mycobacteria.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Barry CE, III, Lee RE, Mdluli K, Sampson AE, Schroeder BG, Slayden RA, Yuan Y. Prog Lipid Res. 1998;37:143–179. - PubMed
-
- Daffé M, Draper P. Adv Microbial Physiol. 1998;39:131–203. - PubMed
-
- Niederweis M. Mol Microbiol. 2003;49:1167–1177. - PubMed
-
- Brennan PJ, Nikaido H. Annu Rev Biochem. 1995;64:29–63. - PubMed
-
- Minnikin D. In: The Biology of Mycobacteria. Tatledge C, Stanford J, editors. Vol 1. London: Academic; 1982. pp. 94–184.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

