Contribution of de novo fatty acid synthesis to hepatic steatosis and insulin resistance: lessons from genetically engineered mice
- PMID: 18317565
- PMCID: PMC2254980
- DOI: 10.1172/JCI34275
Contribution of de novo fatty acid synthesis to hepatic steatosis and insulin resistance: lessons from genetically engineered mice
Abstract
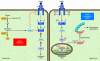
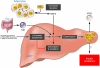
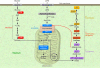
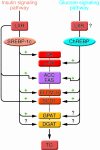
Nonalcoholic fatty liver disease (NAFLD) is associated with obesity, insulin resistance, and type 2 diabetes. NAFLD represents a large spectrum of diseases ranging from (i) fatty liver (hepatic steatosis); (ii) steatosis with inflammation and necrosis; and (iii) cirrhosis. Although the molecular mechanism leading to the development of hepatic steatosis in the pathogenesis of NAFLD is complex, recent animal models have shown that modulating important enzymes in fatty acid synthesis in liver may be key for the treatment of NAFLD. This review discusses recent advances in the field.
Figures
References
-
- Ahmed M.H., Byrne C.D. Modulation of sterol regulatory element binding proteins (SREBPs) as potential treatments for non-alcoholic fatty liver disease (NAFLD). Drug Discov. Today. 2007;12:740–747. - PubMed
-
- Abdelmalek M.F., Diehl A.M. Nonalcoholic fatty liver disease as a complication of insulin resistance. Med. Clin. North Am. 2007;91:1125–1149. - PubMed
-
- Charlton M. Nonalcoholic fatty liver disease: a review of current understanding and future impact. Clin. Gastroenterol. Hepatol. 2004;2:1048–1058. - PubMed
-
- Adams L.A., Lindor K.D. Nonalcoholic fatty liver disease. Ann. Epidemiol. 2007;17:863–869. - PubMed
-
- Yamaguchi K., et al. Inhibiting triglyceride synthesis improves hepatic steatosis but exacerbates liver damage and fibrosis in obese mice with nonalcoholic steatohepatitis. Hepatology. 2007;45:1366–1374. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

