In vivo time-lapse microscopy reveals no loss of murine myonuclei during weeks of muscle atrophy
- PMID: 18317591
- PMCID: PMC2262032
- DOI: 10.1172/JCI34022
In vivo time-lapse microscopy reveals no loss of murine myonuclei during weeks of muscle atrophy
Abstract
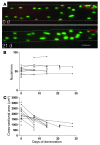
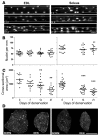
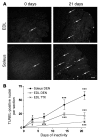
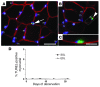
Numerous studies have suggested that muscle atrophy is accompanied by apoptotic loss of myonuclei and therefore recovery would require replenishment by muscle stem cells. We used in vivo time-lapse microscopy to observe the loss and replenishment of myonuclei in murine muscle fibers following induced muscle atrophy. To our surprise, imaging of single fibers for up to 28 days did not support the concept of nuclear loss during atrophy. Muscles were inactivated by denervation, nerve impulse block, or mechanical unloading. Nuclei were stained in vivo either acutely by intracellular injection of fluorescent oligonucleotides or in time-lapse studies after transfection with a plasmid encoding GFP with a nuclear localization signal. We observed no loss of myonuclei in fast- or slow-twitch muscle fibers despite a greater than 50% reduction in fiber cross-sectional area. TUNEL labeling of fragmented DNA on histological sections revealed high levels of apoptotic nuclei in inactive muscles. However, when costained for laminin and dystrophin, virtually none of the TUNEL-positive nuclei could be classified as myonuclei; apoptosis was confined to stromal and satellite cells. We conclude that disuse atrophy is not a degenerative process, but is rather a change in the balance between protein synthesis and proteolysis in a permanent cell syncytium.
Figures