IFN-gamma- and TNF-dependent bystander eradication of antigen-loss variants in established mouse cancers
- PMID: 18317595
- PMCID: PMC2262029
- DOI: 10.1172/JCI33522
IFN-gamma- and TNF-dependent bystander eradication of antigen-loss variants in established mouse cancers
Abstract
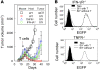
Tumors elicit antitumor immune responses, but over time they evolve and can escape immune control through various mechanisms, including the loss of the antigen to which the response is directed. The escape of antigen-loss variants (ALVs) is a major obstacle to T cell-based immunotherapy for cancer. However, cancers can be cured if both the number of CTLs and the expression of antigen are high enough to allow targeting of not only tumor cells, but also the tumor stroma. Here, we showed that IFN-gamma and TNF produced by CTLs were crucial for the elimination of established mouse tumors, including ALVs. In addition, both BM- and non-BM-derived stromal cells were required to express TNF receptors and IFN-gamma receptors for the elimination of ALVs. Although IFN-gamma and TNF were not required by CTLs for perforin-mediated killing of antigen-expressing tumor cells, the strong inference is that tumor antigen-specific CTLs must secrete IFN-gamma and TNF for destruction of tumor stroma. Therefore, bystander killing of ALVs may result from IFN-gamma and TNF acting on tumor stroma.
Figures

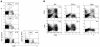


References
-
- Ward P.A., Varani J. Mechanisms of neutrophil-mediated killing of endothelial cells. J. Leukoc. Biol. 1990;48:97–102. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

