Sulfatase 2 up-regulates glypican 3, promotes fibroblast growth factor signaling, and decreases survival in hepatocellular carcinoma
- PMID: 18318435
- PMCID: PMC2536494
- DOI: 10.1002/hep.22202
Sulfatase 2 up-regulates glypican 3, promotes fibroblast growth factor signaling, and decreases survival in hepatocellular carcinoma
Abstract
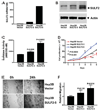
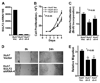
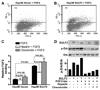
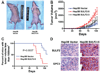
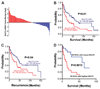
It has been shown that the heparin-degrading endosulfatase, sulfatase 1 (SULF1), functions as a liver tumor suppressor, but the role of the related sulfatase, sulfatase 2 (SULF2), in liver carcinogenesis remains to be elucidated. We investigated the effect of SULF2 on liver tumorigenesis. Expression of SULF2 was increased in 79 (57%) of 139 hepatocellular carcinomas (HCCs) and 8 (73%) of 11 HCC cell lines. Forced expression of SULF2 increased HCC cell growth and migration, whereas knockdown of SULF2 using short hairpin RNA targeting SULF2 abrogated HCC cell proliferation and migration in vitro. Because SULF1 and SULF2 desulfate heparan sulfate proteoglycans (HSPGs) and the HSPG glypican 3 (GPC3) is up-regulated in HCC, we investigated the effects of SULF2 on GPC3 expression and the association of SULF2 with GPC3. SULF2-mediated cell growth was associated with increased binding of fibroblast growth factor 2 (FGF2), phosphorylation of extracellular signal-regulated kinase and AKT, and expression of GPC3. Knockdown of GPC3 attenuated FGF2 binding in SULF2-expressing HCC cells. The effects of SULF2 on up-regulation of GPC3 and tumor growth were confirmed in nude mouse xenografts. Moreover, HCC patients with increased SULF2 expression in resected HCC tissues had a worse prognosis and a higher rate of recurrence after surgery.
Conclusion: In contrast to the tumor suppressor effect of SULF1, SULF2 has an oncogenic effect in HCC mediated in part through up-regulation of FGF signaling and GPC3 expression.
Conflict of interest statement
Potential conflict of interest: Nothing to report.
Figures



References
-
- Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. - PubMed
-
- El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–2576. - PubMed
-
- Roberts LR, Gores GJ. Emerging drugs for hepatocellular carcinoma. Expert Opin Emerg Drugs. 2006;11:469–487. - PubMed
-
- Abou-Alfa GK, Schwartz L, Ricci S, Amadori D, Santoro A, Figer A, et al. Phase II study of sorafenib in patients with advanced hepatocellular carcinoma. J Clin Oncol. 2006;24:4293–4300. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
