Generalized glucocorticoid resistance: clinical aspects, molecular mechanisms, and implications of a rare genetic disorder
- PMID: 18319312
- PMCID: PMC2386273
- DOI: 10.1210/jc.2008-0040
Generalized glucocorticoid resistance: clinical aspects, molecular mechanisms, and implications of a rare genetic disorder
Abstract
Context: Primary generalized glucocorticoid resistance is a rare genetic condition characterized by generalized, partial, target-tissue insensitivity to glucocorticoids. We review the clinical aspects, molecular mechanisms, and implications of this disorder.
Evidence acquisition: We conducted a systematic review of the published, peer-reviewed medical literature using MEDLINE (1975 through February 2008) to identify original articles and reviews on this topic.
Evidence synthesis: We have relied on the experience of a number of experts in the field, including our extensive personal experience.
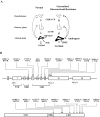
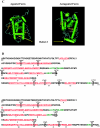
Conclusions: The clinical spectrum of primary generalized glucocorticoid resistance is broad, ranging from asymptomatic to severe cases of hyperandrogenism, fatigue, and/or mineralocorticoid excess. The molecular basis of the condition has been ascribed to mutations in the human glucocorticoid receptor (hGR) gene, which impair glucocorticoid signal transduction and reduce tissue sensitivity to glucocorticoids. A consequent increase in the activity of the hypothalamic-pituitary-adrenal axis compensates for the reduced sensitivity of peripheral tissues to glucocorticoids at the expense of ACTH hypersecretion-related pathology. The study of functional defects of natural hGR mutants enhances our understanding of the molecular mechanisms of hGR action and highlights the importance of integrated cellular and molecular signaling mechanisms for maintaining homeostasis and preserving normal physiology.
Figures

References
-
- Kino T, Chrousos GP 2005 Glucocorticoid effects on gene expression. In: Steckler T, Kalin NH, Reul JMHM, eds. Handbook of stress and the brain. Amsterdam: Elsevier; 295–311
-
- Chrousos GP, Charmandari E, Kino T 2004 Glucocorticoid action networks–an introduction to systems biology. J Clin Endocrinol Metab 89:563–564 - PubMed
-
- Chrousos GP 2004 The glucocorticoid receptor gene, longevity, and the complex disorders of Western societies. Am J Med 117:204–207 - PubMed
-
- Galon J, Franchimont D, Hiroi N, Frey G, Boettner A, Ehrhart-Bornstein M, O'Shea JJ, Chrousos GP, Bornstein SR 2002 Gene profiling reveals unknown enhancing and suppressive actions of glucocorticoids on immune cells. FASEB J 16:61–71 - PubMed
-
- Zhou J, Cidlowski JA 2005 The human glucocorticoid receptor: one gene, multiple proteins and diverse responses. Steroids 70:407–417 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

