Inhibition of mTOR pathway by everolimus cooperates with EGFR inhibitors in human tumours sensitive and resistant to anti-EGFR drugs
- PMID: 18319715
- PMCID: PMC2266842
- DOI: 10.1038/sj.bjc.6604269
Inhibition of mTOR pathway by everolimus cooperates with EGFR inhibitors in human tumours sensitive and resistant to anti-EGFR drugs
Abstract
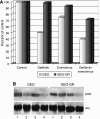
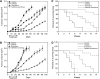
Inhibition of a single transduction pathway is often inefficient due to activation of alternative signalling. The mammalian target of rapamycin (mTOR) is a key intracellular kinase integrating proliferation, survival and angiogenic pathways and has been implicated in the resistance to EGFR inhibitors. Thus, mTOR blockade is pursued to interfere at multiple levels with tumour growth. We used everolimus (RAD001) to inhibit mTOR, alone or in combination with anti-EGFR drugs gefitinib or cetuximab, on human cancer cell lines sensitive and resistant to EGFR inhibitors, both in vitro and in vivo. We demonstrated that everolimus is active against EGFR-resistant cancer cell lines and partially restores the ability of EGFR inhibitors to inhibit growth and survival. Everolimus reduces the expression of EGFR-related signalling effectors and VEGF production, inhibiting proliferation and capillary tube formation of endothelial cells, both alone and in combination with gefitinib. Finally, combination of everolimus and gefitinib inhibits growth of GEO and GEO-GR (gefitinib resistant) colon cancer xenografts, activation of signalling proteins and VEGF secretion. Targeting mTOR pathway with everolimus overcomes resistance to EGFR inhibitors and produces a cooperative effect with EGFR inhibitors, providing a valid therapeutic strategy to be tested in a clinical setting.
Figures



References
-
- Abraham RT, Gibbons JJ (2007) The mammalian target of rapamycin signaling pathway: twists and turns in the road to cancer therapy. Clin Cancer Res 13: 3109–3114 - PubMed
-
- Adjei AA (2006) Novel combinations based on epidermal growth factor receptor inhibition. Clin Cancer Res 12: 4446s–4450s - PubMed
-
- Baselga J, Arteaga CL (2005) Critical update and emerging trends in epidermal growth factor receptor targeting in cancer. J Clin Oncol 23: 2445–2459 - PubMed
-
- Bezjak A, Tu D, Seymour L, Clark G, Trajkovic A, Zukin M, Ayoub J, Lago S, de Albuquerque Ribeiro R, Gerogianni A, Cyjon A, Noble J, Laberge F, Chan RT, Fenton D, von Pawel J, Reck M, Shepherd FA (2006) Symptom improvement in lung cancer patients treated with erlotinib: quality of life analysis of the National Cancer Institute of Canada Clinical Trials Group Study BR.21. J Clin Oncol 24: 3831–3837 - PubMed
-
- Bianco R, Shin I, Ritter CA, Yakes FM, Basso A, Rosen N, Tsurutani N, Dennis PA, Mills GB, Arteaga CL (2003) Loss of PTEN/MMAC1/TEP in EGF receptor-expressing tumor cells counteracts the antitumor action of EGFR tyrosine kinase inhibitors. Oncogene 22: 2812–2822 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

