Inhibition of JAK1, 2/STAT3 signaling induces apoptosis, cell cycle arrest, and reduces tumor cell invasion in colorectal cancer cells
- PMID: 18320073
- PMCID: PMC2259457
- DOI: 10.1593/neo.07971
Inhibition of JAK1, 2/STAT3 signaling induces apoptosis, cell cycle arrest, and reduces tumor cell invasion in colorectal cancer cells
Abstract
Abnormalities in the STAT3 pathway are involved in the oncogenesis of several cancers. However, the mechanism by which dysregulated STAT3 signaling contributes to the progression of human colorectal cancer (CRC) has not been elucidated, nor has the role of JAK, the physiological activator of STAT3, been evaluated. To investigate the role of both JAK and STAT3 in CRC progression, we inhibited JAK with AG490 and depleted STAT3 with a SiRNA. Our results demonstrate that STAT3 and both JAK1 and 2 are involved in CRC cell growth, survival, invasion, and migration through regulation of gene expression, such as Bcl-2, p1(6ink4a), p21(waf1/cip1), p27(kip1), E-cadherin, VEGF, and MMPs. Importantly, the FAK is not required for STAT3-mediated regulation, but does function downstream of JAK. In addition, our data show that proteasome-mediated proteolysis promotes dephosphorylation of the JAK2, and consequently, negatively regulates STAT3 signaling in CRC. Moreover, immunohistochemical staining reveals that nuclear staining of phospho-STAT3 mostly presents in adenomas and adenocarcinomas, and a positive correlation is found between phospho-JAK2 immunoreactivity and the differentiation of colorectal adenocarcinomas. Therefore, our findings illustrate the biologic significance of JAK1, 2/STAT3 signaling in CRC progression and provide novel evidence that the JAK/STAT3 pathway may be a new potential target for therapy of CRC.
Figures
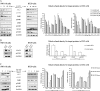


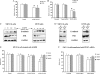
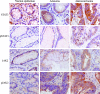
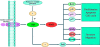
References
-
- Niwa Y, Kanda H, Shikauchi Y, Saiura A, Matsubara K, Kitagawa T, Yamamoto J, Kubo T, Yoshikawa H. Methylation silencing of SOCS-3 promotes cell growth and migration by enhancing JAK/STAT and FAK signalings in human hepatocellular carcinoma. Oncogene. 2005;24:6406–6417. - PubMed
-
- Lacronique V, Boureux A, Valle VD, Poirel H, Quang CT, Mauchauffe M, Berthou C, Lessard M, Berger R, Ghysdael J, et al. A TEL-JAK2 fusion protein with constitutive kinase activity in human leukemia. Science. 1997;278:1309–1312. - PubMed
-
- Zhang F, Li C, Halfter H, Liu J. Delineating an oncostatin M-activated STAT3 signaling pathway that coordinates the expression of genes involved in cell cycle regulation and extracellular matrix deposition of MCF-7 cells. Oncogene. 2003;22:894–905. - PubMed
-
- Alvarez JV, Greulich H, Sellers WR, Meyerson M, Frank DA. Signal transducer and activator of transcription 3 is required for the oncogenic effects of non-small-cell lung cancer-associated mutations of the epidermal growth factor receptor. Cancer Res. 2006;66:3162–3168. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
