Alanyl-glutamine and glutamine supplementation improves 5-fluorouracil-induced intestinal epithelium damage in vitro
- PMID: 18320312
- PMCID: PMC4003886
- DOI: 10.1007/s10620-008-0215-0
Alanyl-glutamine and glutamine supplementation improves 5-fluorouracil-induced intestinal epithelium damage in vitro
Abstract
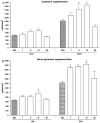
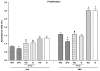
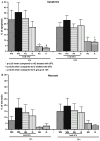
In this study, we have examined the role of glutamine derivatives in reducing 5-fluorouracil (5-FU)-induced epithelial damage in an undifferentiated crypt intestinal cell line, IEC-6. In this model, we have investigated proliferation indirectly by detecting the enzyme-derived formazan dye from the tetrazolium salt WST-1 in viable cells at 24 and 48 h after 5-FU treatment. Migration was measured at 12 and 24 h after razor scraping of the cell monolayer. Cell death was measured by quantifying the percentage of apoptotic and necrotic figures by flow cytometry at 12 and 24 h following 5-FU challenge. Neither glutamine nor alanyl-glutamine prevented 5-FU-induced apoptosis and necrosis in IEC-6 cells at 12 and 24 h after 5-FU challenge. However, glutamine and alanyl-glutamine enhanced migration and proliferation when compared with 5-FU-treated controls (P < 0.05). These new findings support our earlier study on the benefit of oral glutamine in enhancing epithelial recovery after 5-FU challenge.
Figures



Similar articles
-
Alanyl-glutamine attenuates 5-fluorouracil-induced intestinal mucositis in apolipoprotein E-deficient mice.Braz J Med Biol Res. 2015 Jun;48(6):493-501. doi: 10.1590/1414-431X20144360. Epub 2015 Apr 28. Braz J Med Biol Res. 2015. PMID: 25945744 Free PMC article.
-
Alanyl-glutamine hastens morphologic recovery from 5-fluorouracil-induced mucositis in mice.Nutrition. 2004 Oct;20(10):934-41. doi: 10.1016/j.nut.2004.06.016. Nutrition. 2004. PMID: 15474885
-
Protective effects of alanyl-glutamine supplementation against nelfinavir-induced epithelial impairment in IEC-6 cells and in mouse intestinal mucosa.Cancer Biol Ther. 2012 Dec;13(14):1482-90. doi: 10.4161/cbt.22251. Epub 2012 Sep 17. Cancer Biol Ther. 2012. PMID: 22986234 Free PMC article.
-
Stimulation of intestinal epithelial restitution by prostaglandin E(1) analogue.Cancer Chemother Pharmacol. 2003 Mar;51(3):216-20. doi: 10.1007/s00280-003-0576-1. Epub 2003 Feb 26. Cancer Chemother Pharmacol. 2003. PMID: 12655439
-
Clostridium difficile toxin A induces intestinal epithelial cell apoptosis and damage: role of Gln and Ala-Gln in toxin A effects.Dig Dis Sci. 2005 Jul;50(7):1271-8. doi: 10.1007/s10620-005-2771-x. Dig Dis Sci. 2005. PMID: 16047471
Cited by
-
A diet containing whey protein, glutamine, and TGFbeta modulates gut protein metabolism during chemotherapy-induced mucositis in rats.Dig Dis Sci. 2010 Aug;55(8):2172-81. doi: 10.1007/s10620-009-1039-2. Epub 2009 Nov 13. Dig Dis Sci. 2010. PMID: 19911274
-
Stem Cell Intrinsic Hexosamine Metabolism Regulates Intestinal Adaptation to Nutrient Content.Dev Cell. 2018 Oct 8;47(1):112-121.e3. doi: 10.1016/j.devcel.2018.08.011. Epub 2018 Sep 13. Dev Cell. 2018. PMID: 30220570 Free PMC article.
-
Novel in vitro and in vivo models and potential new therapeutics to break the vicious cycle of Cryptosporidium infection and malnutrition.J Infect Dis. 2012 May 1;205(9):1464-71. doi: 10.1093/infdis/jis216. Epub 2012 Mar 26. J Infect Dis. 2012. PMID: 22454464 Free PMC article.
-
Targeting the human microbiome with antibiotics, probiotics, and prebiotics: gastroenterology enters the metagenomics era.Gastroenterology. 2009 May;136(6):2015-31. doi: 10.1053/j.gastro.2009.01.072. Gastroenterology. 2009. PMID: 19462507 Free PMC article. Review.
-
Intestinal epithelial restitution after TcdB challenge and recovery from Clostridium difficile infection in mice with alanyl-glutamine treatment.J Infect Dis. 2013 May 15;207(10):1505-15. doi: 10.1093/infdis/jit041. Epub 2013 Jan 28. J Infect Dis. 2013. PMID: 23359592 Free PMC article.
References
-
- Potten CS. Kinetics and possible regulation of crypt cell populations under normal and stress conditions. Bull Cancer. 1975;62:419–430. - PubMed
-
- Potten CS. Cell cycles in cell hierarchies. Int J Radiat Biol Relat Stud Phys Chem Med. 1986;49:257–278. - PubMed
-
- Potten CS, Booth C. The role of radiation-induced and spontaneous apoptosis in the homeostasis of the gastrointestinal epithelium: a brief review. Comp Biochem Physiol B Biochem Mol Biol. 1997;118:473–478. - PubMed
-
- Clatworthy JP, Subramanian V. Stem cells and the regulation of proliferation, differentiation and patterning in the intestinal epithelium: emerging insights from gene expression patterns, transgenic and gene ablation studies. Mech Dev. 2001;101:3–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

