Dopaminergic modulation of auditory cortex-dependent memory consolidation through mTOR
- PMID: 18321872
- PMCID: PMC2567422
- DOI: 10.1093/cercor/bhn026
Dopaminergic modulation of auditory cortex-dependent memory consolidation through mTOR
Abstract
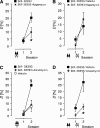
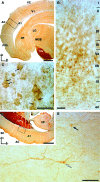
Previous studies in the auditory cortex of Mongolian gerbils on discrimination learning of the direction of frequency-modulated tones (FMs) revealed that long-term memory formation involves activation of the dopaminergic system, activity of the protein kinase mammalian target of rapamycin (mTOR), and protein synthesis. This led to the hypothesis that the dopaminergic system might modulate memory formation via regulation of mTOR, which is implicated in translational control. Here, we report that the D1/D5 dopamine receptor agonist SKF-38393 substantially improved gerbils' FM discrimination learning when administered systemically or locally into the auditory cortex shortly before, shortly after, or 1 day before conditioning. Although acquisition performance during initial training was normal, the discrimination of FMs was enhanced during retraining performed hours or days after agonist injection compared with vehicle-injected controls. The D1/D5 receptor antagonist SCH-23390, the mTOR inhibitor rapamycin, and the protein synthesis blocker anisomycin suppressed this effect. By immunohistochemistry, D1 dopamine receptors were identified in the gerbil auditory cortex predominantly in the infragranular layers. Together, these findings suggest that in the gerbil auditory cortex dopaminergic inputs regulate mTOR-mediated, protein synthesis-dependent mechanisms, thus controlling for hours or days the consolidation of memory required for the discrimination of complex auditory stimuli.
Figures


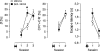

References
-
- Abel T, Lattal KM. Molecular mechanisms of memory acquisition, consolidation and retrieval. Curr Opin Neurobiol. 2001;11:180–187. - PubMed
-
- Aitkin L. The auditory cortex. London: Chapman and Hall; 1990.
-
- Atzori M, Kanold PO, Pineda JC, Flores-Hernandez J, Paz RD. Dopamine prevents muscarinic-induced decrease of glutamate release in the auditory cortex. Neuroscience. 2005;134:1153–1165. - PubMed
-
- Bao S, Chan VT, Merzenich MM. Cortical remodelling induced by activity of ventral tegmental dopamine neurons. Nature. 2001;412:79–83. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

