Casein kinase II motif-dependent phosphorylation of human papillomavirus E7 protein promotes p130 degradation and S-phase induction in differentiated human keratinocytes
- PMID: 18321970
- PMCID: PMC2346766
- DOI: 10.1128/JVI.01202-07
Casein kinase II motif-dependent phosphorylation of human papillomavirus E7 protein promotes p130 degradation and S-phase induction in differentiated human keratinocytes
Abstract
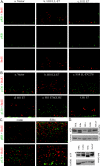
The E7 proteins of human papillomaviruses (HPVs) promote S-phase reentry in differentiated keratinocytes of the squamous epithelia to support viral DNA amplification. In this study, we showed that nuclear p130 was present in the differentiated strata of several native squamous epithelia susceptible to HPV infection. In contrast, p130 was below the level of detection in HPV-infected patient specimens. In submerged and organotypic cultures of primary human keratinocytes, the E7 proteins of the high-risk mucosotrophic HPV-18, the benign cutaneous HPV-1, and, to a lesser extent, the low-risk mucosotropic HPV-11 destabilized p130. This E7 activity depends on an intact pocket protein binding domain and a casein kinase II (CKII) phosphorylation motif. Coimmunoprecipitation experiments showed that both E7 domains were important for binding to p130 in extracts of organotypic cultures. Metabolic labeling in vivo demonstrated that E7 proteins were indeed phosphorylated in a CKII motif-dependent manner. Moreover, the efficiencies of the E7 proteins of various HPV types or mutations to induce S-phase reentry in spinous cells correlated with their relative abilities to bind and to destabilize p130. Collectively, these data support the notion that p130 controls the homeostasis of the differentiated keratinocytes and is therefore targeted by E7 for degradation to establish conditions permissive for viral DNA amplification.
Figures
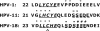




References
-
- Alunni-Fabbroni, M., T. Littlewood, L. Deleu, S. Caldeira, M. Giarre, M. Dell' Orco, and M. Tommasino. 2000. Induction of S phase and apoptosis by the human papillomavirus type 16 E7 protein are separable events in immortalized rodent fibroblasts. Oncogene 192277-2285. - PubMed
-
- Baker, G. L., M. W. Landis, and P. W. Hinds. 2005. Multiple functions of D-type cyclins can antagonize pRb-mediated suppression of proliferation. Cell Cycle 4330-338. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

