Heterogeneity of human macrophages in culture and in atherosclerotic plaques
- PMID: 18321997
- PMCID: PMC2276432
- DOI: 10.2353/ajpath.2008.070513
Heterogeneity of human macrophages in culture and in atherosclerotic plaques
Abstract
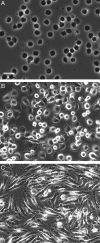
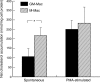
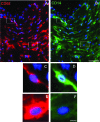
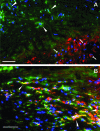
Research suggests that monocytes differentiate into unique lineage-determined macrophage subpopulations in response to the local cytokine environment. The present study evaluated the atherogenic potential of two divergent lineage-determined human monocyte-derived macrophage subpopulations. Monocytes were differentiated for 7 days in the presence of alternative macrophage development cytokines: granulocyte-macrophage colony-stimulating factor to produce granulocyte-macrophage-CSF macrophages (GM-Mac), or macrophage colony-stimulating factor (M-CSF) to produce M-Mac. Gene chip analyses of three monocyte donors demonstrated differential expression of inflammatory and cholesterol homeostasis genes in the macrophage subpopulations. Quantitative PCR confirmed a fivefold elevation in the expression of genes that promote reverse cholesterol transport (PPAR-gamma, LXR-alpha, and ABCG1) and macrophage emigration from lesions (CCR7) in GM-Mac compared to that in M-Mac. Immunocytochemistry confirmed enhanced expression of the proinflammatory marker CD14 in M-Mac relative to GM-Mac. M-Mac spontaneously accumulated cholesterol when incubated with unmodified low-density lipoprotein whereas GM-Mac only accumulated similar levels of cholesterol after protein kinase C activation. Immunostained human coronary arteries showed that macrophages with similar antigen expression to that of M-Mac (CD68(+)/CD14(+)) were predominant within atherosclerotic lesions whereas macrophages with antigen expression similar to GM-Mac (CD68(+)/CD14(-)) were predominant in areas devoid of disease. The identification of macrophage subpopulations with different gene expression patterns and, thus, different potentials for promoting atherosclerosis has important experimental and clinical implications and could prove to be a valuable finding in developing therapeutic interventions in diseases dependent on macrophage function.
Figures




References
-
- Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;362:801–809. - PubMed
-
- Shah PK, Falk E, Badimon JJ, Fernandez-Ortiz A, Mailhac A, Villareal-Levy G, Fallon JT, Regnstrom J, Fuster V. Human monocyte-derived macrophages induce collagen breakdown in fibrous caps of atherosclerotic plaques. Potential role of matrix-degrading metalloproteinases and implications for plaque rupture. Circulation. 1995;92:1565–1569. - PubMed
-
- Crawley J, Lupu F, Westmuckett AD, Severs NJ, Kakkar VV, Lupu C. Expression, localization, and activity of tissue factor pathway inhibitor in normal and atherosclerotic human vessels. Arterioscler Thromb Vasc Biol. 2000;20:1362–1373. - PubMed
-
- Wintergerst ES, Jelk J, Asmis R. Differential expression of CD14. CD36 and the LDL receptor on human monocyte-derived macrophages A novel cell culture system to study macrophage differentiation and heterogeneity. Histochem Cell Biol. 1998;110:231–241. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

