Txnip balances metabolic and growth signaling via PTEN disulfide reduction
- PMID: 18322014
- PMCID: PMC2268825
- DOI: 10.1073/pnas.0800293105
Txnip balances metabolic and growth signaling via PTEN disulfide reduction
Abstract
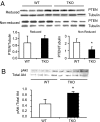
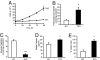
Thioredoxin-interacting protein (Txnip) inhibits thioredoxin NADPH-dependent reduction of protein disulfides. Total Txnip knockout (TKO) mice adapted inappropriately to prolonged fasting by shifting fuel dependence of skeletal muscle and heart from fat and ketone bodies to glucose. TKO mice exhibited increased Akt signaling, insulin sensitivity, and glycolysis in oxidative tissues (skeletal muscle and hearts) but not in lipogenic tissues (liver and adipose tissue). The selective activation of Akt in skeletal muscle and hearts was associated with impaired mitochondrial fuel oxidation and the accumulation of oxidized (inactive) PTEN, whose activity depends on reduction of two critical cysteine residues. Whereas muscle- and heart-specific Txnip knockout mice recapitulated the metabolic phenotype exhibited by TKO mice, liver-specific Txnip knockout mice were similar to WT mice. Embryonic fibroblasts derived from knockout mice also accumulated oxidized (inactive) PTEN and had elevated Akt phosphorylation. In addition, they had faster growth rates and increased dependence on anaerobic glycolysis due to impaired mitochondrial fuel oxidation, and they were resistant to doxorubicin-facilitated respiration-dependent apoptosis. In the absence of Txnip, oxidative inactivation of PTEN and subsequent activation of Akt attenuated mitochondrial respiration, resulting in the accumulation of NADH, a competitive inhibitor of thioredoxin NADPH-reductive activation of PTEN. These findings indicate that, in nonlipogenic tissues, Txnip is required to maintain sufficient thioredoxin NADPH activity to reductively reactivate oxidized PTEN and oppose Akt downstream signaling.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
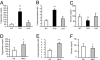



References
-
- Maehama T, Dixon JE. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem. 1998;273:13375–13378. - PubMed
-
- Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–1657. - PubMed
-
- Meuillet EJ, Mahadevan D, Berggren M, Coon A, Powis G. Thioredoxin-1 binds to the C2 domain of PTEN inhibiting PTEN′s lipid phosphatase activity and membrane binding: A mechanism for the functional loss of PTEN′s tumor suppressor activity. Arch Biochem Biophys. 2004;429:123–133. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

