Transient receptor potential A1 is a sensory receptor for multiple products of oxidative stress
- PMID: 18322093
- PMCID: PMC2709206
- DOI: 10.1523/JNEUROSCI.5369-07.2008
Transient receptor potential A1 is a sensory receptor for multiple products of oxidative stress
Abstract
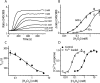
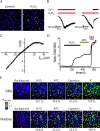
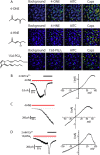
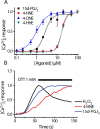
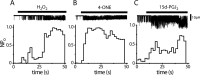
Transient receptor potential A1 (TRPA1) is expressed in a subset of nociceptive sensory neurons where it acts as a sensor for environmental irritants, including acrolein, and some pungent plant ingredients such as allyl isothiocyanate and cinnamaldehyde. These exogenous compounds activate TRPA1 by covalent modification of cysteine residues. We have used electrophysiological methods and measurements of intracellular calcium concentration ([Ca(2+)](i)) to show that TRPA1 is activated by several classes of endogenous thiol-reactive molecules. TRPA1 was activated by hydrogen peroxide (H(2)O(2); EC(50), 230 microM), by endogenously occurring alkenyl aldehydes (EC(50): 4-hydroxynonenal 19.9 microM, 4-oxo-nonenal 1.9 microM, 4-hydroxyhexenal 38.9 microM) and by the cyclopentenone prostaglandin, 15-deoxy-delta(12,14)-prostaglandin J(2) (15d-PGJ(2), EC(50): 5.6 microM). The effect of H(2)O(2) was reversed by treatment with dithiothreitol indicating that H(2)O(2) acts by promoting the formation of disulfide bonds whereas the actions of the alkenyl aldehydes and 15d-PGJ(2) were not reversed, suggesting that these agents form Michael adducts. H(2)O(2) and the naturally occurring alkenyl aldehydes and 15d-PGJ(2) acted on a subset of isolated rat and mouse sensory neurons [approximately 25% of rat dorsal root ganglion (DRG) and approximately 50% of nodose ganglion neurons] to evoke a depolarizing inward current and an increase in [Ca(2+)](i) in TRPA1 expressing neurons. The abilities of H(2)O(2), alkenyl aldehydes and 15d-PGJ(2) to raise [Ca(2+)](i) in mouse DRG neurons were greatly reduced in neurons from trpa1(-/-) mice. Furthermore, intraplantar injection of either H(2)O(2) or 15d-PGJ2 evoked a nocifensive/pain response in wild-type mice, but not in trpa1(-/-) mice. These data demonstrate that multiple agents produced during episodes of oxidative stress can activate TRPA1 expressed in sensory neurons.
Figures



References
-
- Bandell M, Story GM, Hwang SW, Viswanath V, Eid SR, Petrus MJ, Earley TJ, Patapoutian A. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron. 2004;41:849–857. - PubMed
-
- Bautista DM, Jordt SE, Nikai T, Tsuruda PR, Read AJ, Poblete J, Yamoah EN, Basbaum AI, Julius D. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell. 2006;124:1269–1282. - PubMed
-
- Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
