Interplay between cyclin-dependent kinase 5 and glycogen synthase kinase 3 beta mediated by neuregulin signaling leads to differential effects on tau phosphorylation and amyloid precursor protein processing
- PMID: 18322105
- PMCID: PMC6671193
- DOI: 10.1523/JNEUROSCI.5245-07.2008
Interplay between cyclin-dependent kinase 5 and glycogen synthase kinase 3 beta mediated by neuregulin signaling leads to differential effects on tau phosphorylation and amyloid precursor protein processing
Abstract
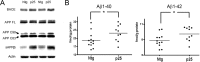
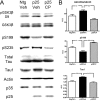
Cyclin-dependent kinase 5 (cdk5) and glycogen synthase kinase 3beta (GSK3beta) have been implicated in pathogenic processes associated with Alzheimer's disease because both kinases regulate tau hyperphosphorylation and enhance amyloid precursor protein (APP) processing leading to an increase in amyloid beta (Abeta) production. Here we show that young p25 overexpressing mice have enhanced cdk5 activity but reduced GSK3beta activity attributable to phosphorylation at the inhibitory GSK3beta-serine 9 (GSK3beta-S9) site. Phosphorylation at this site was mediated by enhanced activity of the neuregulin receptor complex, ErbB, and activation of the downstream phosphatidylinositol 3 kinase/Akt pathway. Young p25 mice had elevated Abeta peptide levels, but phospho-tau levels were decreased overall. Thus, cdk5 appears to play a dominant role in the regulation of amyloidogenic APP processing, whereas GSK3beta plays a dominant role in overall tau phosphorylation. In older mice, GSK3beta inhibitory phosphorylation at S9 was reduced relative to young mice. Abeta peptide levels were still elevated but phospho-tau levels were either unchanged or showed a trend to increase, suggesting that GSK3beta activity increases with aging. Inhibition of cdk5 by a specific inhibitor reduced cdk5 activity in p25 mice, leading to reduced Abeta production in both young and old mice. However, in young mice, cdk5 inhibition reversed GSK3beta inhibition, leading to an increase in overall tau phosphorylation. When cdk5 inhibitor was administered to very old, nontransgenic mice, inhibition of cdk5 reduced Abeta levels, and phospho-tau levels showed a trend to increase. Thus, cdk5 inhibitors may not be effective in targeting tau phosphorylation in the elderly.
Figures







Similar articles
-
Suppression of cyclin-dependent kinase 5 activation by amyloid precursor protein: a novel excitoprotective mechanism involving modulation of tau phosphorylation.J Neurosci. 2005 Dec 14;25(50):11542-52. doi: 10.1523/JNEUROSCI.3831-05.2005. J Neurosci. 2005. PMID: 16354912 Free PMC article.
-
CDK5 activator protein p25 preferentially binds and activates GSK3β.Proc Natl Acad Sci U S A. 2014 Nov 11;111(45):E4887-95. doi: 10.1073/pnas.1402627111. Epub 2014 Oct 20. Proc Natl Acad Sci U S A. 2014. PMID: 25331900 Free PMC article.
-
Neuroprotective effects of regulators of the glycogen synthase kinase-3beta signaling pathway in a transgenic model of Alzheimer's disease are associated with reduced amyloid precursor protein phosphorylation.J Neurosci. 2007 Feb 21;27(8):1981-91. doi: 10.1523/JNEUROSCI.4321-06.2007. J Neurosci. 2007. PMID: 17314294 Free PMC article.
-
Deregulated Cdk5 activity is involved in inducing Alzheimer's disease.Arch Med Res. 2012 Nov;43(8):655-62. doi: 10.1016/j.arcmed.2012.10.015. Epub 2012 Nov 7. Arch Med Res. 2012. PMID: 23142263 Free PMC article. Review.
-
Untangling tau hyperphosphorylation in drug design for neurodegenerative diseases.Nat Rev Drug Discov. 2007 Jun;6(6):464-79. doi: 10.1038/nrd2111. Nat Rev Drug Discov. 2007. PMID: 17541419 Review.
Cited by
-
Specific inhibition of p25/Cdk5 activity by the Cdk5 inhibitory peptide reduces neurodegeneration in vivo.J Neurosci. 2013 Jan 2;33(1):334-43. doi: 10.1523/JNEUROSCI.3593-12.2013. J Neurosci. 2013. PMID: 23283346 Free PMC article.
-
Mice lacking phosphatase PP2A subunit PR61/B'delta (Ppp2r5d) develop spatially restricted tauopathy by deregulation of CDK5 and GSK3beta.Proc Natl Acad Sci U S A. 2011 Apr 26;108(17):6957-62. doi: 10.1073/pnas.1018777108. Epub 2011 Apr 11. Proc Natl Acad Sci U S A. 2011. PMID: 21482799 Free PMC article.
-
Therapeutic strategies for Alzheimer's disease.Mol Neurobiol. 2008 Apr-Jun;37(2-3):171-86. doi: 10.1007/s12035-008-8031-2. Epub 2008 Jun 26. Mol Neurobiol. 2008. PMID: 18581273 Review.
-
Remimazolam induced cognitive dysfunction in mice via glutamate excitotoxicity.Transl Neurosci. 2022 May 31;13(1):104-115. doi: 10.1515/tnsci-2022-0220. eCollection 2022 Jan 1. Transl Neurosci. 2022. PMID: 35734308 Free PMC article.
-
Post-Translational Modifications in Tau and Their Roles in Alzheimer's Pathology.Curr Alzheimer Res. 2024;21(1):24-49. doi: 10.2174/0115672050301407240408033046. Curr Alzheimer Res. 2024. PMID: 38623984 Review.
References
-
- Ahlijanian MK, Barrezueta NX, Williams RD, Jakowski A, Kowsz KP, McCarthy S, Coskran T, Carlo A, Seymour PA, Burkhardt JE, Nelson RB, McNeish JD. Hyperphosphorylated tau and neurofilament and cytoskeletal disruptions in mice overexpressing human p25, an activator of cdk5. Proc Natl Acad Sci USA. 2000;97:2910–2915. - PMC - PubMed
-
- Alessi DR, James SR, Downes CP, Holmes AB, Gaffney PR, Reese CB, Cohen P. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Balpha. Curr Biol. 1997;7:261–269. - PubMed
-
- Cadigan KM, Nusse R. Wnt signaling: a common theme in animal development. Genes Dev. 1997;11:3286–3305. - PubMed
-
- Carraway KL, III, Burden SJ. Neuregulins and their receptors. Curr Opin Neurobiol. 1995;5:606–612. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
