Deviation from a strong Th1-dominated to a modest Th17-dominated CD4 T cell response in the absence of IL-12p40 and type I IFNs sustains protective CD8 T cells
- PMID: 18322221
- PMCID: PMC2677099
- DOI: 10.4049/jimmunol.180.6.4109
Deviation from a strong Th1-dominated to a modest Th17-dominated CD4 T cell response in the absence of IL-12p40 and type I IFNs sustains protective CD8 T cells
Abstract
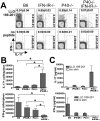
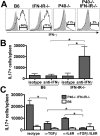
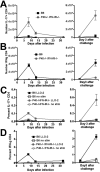
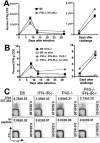
The differentiation of naive CD4 T cells into specific effector subsets is controlled in large part by the milieu of cytokines present during their initial encounter with Ag. Cytokines that drive differentiation of the newly described Th17 lineage have been characterized in vitro, but the cytokines that prime commitment to this lineage in response to infection in vivo are less clear. Listeria monocytogenes (Lm) induces a strong Th1 response in wild-type mice. By contrast, we demonstrate that in the absence of IL-12p40 (or IFN-gamma) and type I IFN receptor signaling, the Th1 Ag-specific CD4 T cell response is virtually abolished and replaced by a relatively low magnitude Th17-dominated response. This Th17 response was dependent on TGF-beta and IL-6. Despite this change in CD4 T cell response, neither the kinetics of the CD4 and CD8 T cell responses, the quality of the CD8 T cell response, nor the ability of CD8 T cells to mediate protection were affected. Thus, generation of protective CD8 T cell immunity was resilient to perturbations that replace a strong Th1-dominated to a reduced magnitude Th17-dominated Ag-specific CD4 T cell response.
Figures

References
-
- Bettelli E, Oukka M, Kuchroo VK. T(H)-17 cells in the circle of immunity and autoimmunity. Nat Immunol. 2007;8:345–350. - PubMed
-
- Weaver CT, Hatton RD, Mangan PR, Harrington LE. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu Rev Immunol. 2007;25:821–852. - PubMed
-
- Mangan PR, Harrington LE, O'Quinn DB, Helms WS, Bullard DC, Elson CO, Hatton RD, Wahl SM, Schoeb TR, Weaver CT. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231–234. - PubMed
-
- Ye P, Rodriguez FH, Kanaly S, Stocking KL, Schurr J, Schwarzenberger P, Oliver P, Huang W, Zhang P, Zhang J, Shellito JE, Bagby GJ, Nelson S, Charrier K, Peschon JJ, Kolls JK. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J Exp Med. 2001;194:519–527. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

