Percutaneous cryosurgery for the treatment of hepatic colorectal metastases
- PMID: 18322961
- PMCID: PMC2693695
- DOI: 10.3748/wjg.14.1430
Percutaneous cryosurgery for the treatment of hepatic colorectal metastases
Abstract
Aim: To determine the safety and efficacy of efficacy of percutaneous cryosurgery for treatment of patients with hepatic colorectal metastases.
Methods: Three hundred and twenty-six patients with non-resectable hepatic colorectal metastases underwent percutaneous cryosurgery under the guidance of ultrasound or CT. Follow-up was 1 mo after cryosurgery and then every 4 mo thereafter by assessment of tumor markers, liver ultrasonography, and abdominal CT. For lesions suspicious of recurrence, a liver biopsy was performed and subsequent repeat cryosurgery was given if histology was positive for cancer.
Results: All patients underwent a total of 526 procedures of cryosurgery. There were 151 patients who underwent repeat procedures of cryosurgery for recurrent tumors in the liver and extrahepatic places. At 3 mo after cryosurgery, carcinoembryonic antigen (CEA) levels in 197 (77.5%) patients who had elevated markers before cryosurgery decreased to normal range. Among 280 patients who received CT following-up, cryotreated lesions showed complete response (CR) in 41 patients (14.6%), partial response (PR) in 115 patients (41.1%), stable disease (SD) in 68 patients (24.3%) and progressive disease (PD) in 56 patients (20%). The recurrence rate was 47.2% during a median follow-up of 32 mo (range, 7-61). Sixty one percent of the recurrences were seen in liver only and 13.9% in liver and extrahepatic areas. The recurrence rate at cryotreated site was only 6.4% for all cases. During a median follow-up of 36 mo (7-62 mo), the median survival of all patient was 29 mo (range 3-62 mo). Overall survival was 78%, 62%, 41%, 34% and 23% at 1, 2, 3, 4 and 5 years, respectively, after the treatment. Patients with tumor size less than 3 cm, tumor in right lobe of liver, lower CEA levels (<100 ng/dL) and post-cryosurgery TACE had higher survival rate. There was no significant difference in terms of survival based on the number of tumors, pre-cryosurgery chemotherapy and the timing of the development of metastases (synchronous vs metachronous). Patients who underwent 2-3 procedures of cryosurgery had increased survival compared to patients who received cryosurgery once only. There was no intra-cryosurgery mortality. Main adverse effects, such as hepatic bleeding, cryoshock, biliary fistula, liver failure, renal insufficiency and liver abscess were only observed in 0.3%-1.5% of patients.
Conclusion: Percutaneous cryosurgery was a safe modality for hepatic colorectal metastases. Rather than an alternative to resection, this technique should be regarded as a complement to hepatectomy and as an additional means of achieving tumor eradication when total excision is not possible.
Figures
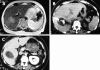
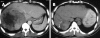

References
-
- Scheele J, Stangl R, Altendorf-Hofmann A. Hepatic metastases from colorectal carcinoma: Impact of surgical resection on the natural history. Br J Surg. 1990;77:1241–1246. - PubMed
-
- Hughes KS, Simon R, Songhorabodi S, Adson MA, Ilstrup DM, Fortner JG, Maclean BJ, Foster JH, Daly JM, Fitzherbert D. Resection of the liver for colorectal carcinoma metastases: a multi-institutional study of patterns of recurrence. Surgery. 1986;100:278–284. - PubMed
-
- Fong Y, Cohen AM, Fortner JG, Enker WE, Turnbull AD, Coit DG, Marrero AM, Prasad M, Blumgart LH, Brennan MF. Liver resection for colorectal metastases. J Clin Oncol. 1997;15:938–946. - PubMed
-
- Moug SJ, Horgan PG. The role of synchronous procedures in the treatment of colorectal liver metastases. Surg Oncol. 2007;16:53–58. - PubMed
-
- Cummings LC, Payes JD, Cooper GS. Survival after hepatic resection in metastatic colorectal cancer: a population-based study. Cancer. 2007;109:718–726. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

