Dual-modality monitoring of targeted intraarterial delivery of mesenchymal stem cells after transient ischemia
- PMID: 18323495
- PMCID: PMC2857730
- DOI: 10.1161/STROKEAHA.107.502047
Dual-modality monitoring of targeted intraarterial delivery of mesenchymal stem cells after transient ischemia
Abstract
Background and purpose: In animal models of stroke, functional improvement has been obtained after stem cell transplantation. Successful therapy depends largely on achieving a robust and targeted cell engraftment, with intraarterial (IA) injection being a potentially attractive route of administration. We assessed the suitability of laser Doppler flow (LDF) signal measurements and magnetic resonance (MR) imaging for noninvasive dual monitoring of targeted IA cell delivery.
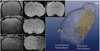
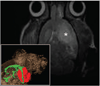
Methods: Transient cerebral ischemia was induced in adult Wistar rats (n=25) followed by IA or intravenous (IV) injection of mesenchymal stem cells (MSCs) labeled with superparamagnetic iron oxide. Cell infusion was monitored in real time with transcranial laser Doppler flowmetry while cellular delivery was assessed with MRI in vivo (4.7 T) and ex vivo (9.4 T).
Results: Successful delivery of magnetically labeled MSCs could be readily visualized with MRI after IA but not IV injection. IA stem cell injection during acute stroke resulted in a high variability of cerebral engraftment. The amount of LDF reduction during cell infusion (up to 80%) was found to correlate well with the degree of intracerebral engraftment, with low LDF values being associated with significant morbidity.
Conclusions: High cerebral engraftment rates are associated with impeded cerebral blood flow. Noninvasive dual-modality imaging enables monitoring of targeted cell delivery, and through interactive adjustment may improve the safety and efficacy of stem cell therapy.
Figures



References
-
- Bjorklund A, Stenevi U. Reconstruction of the nigrostriatal dopamine pathway by intracerebral nigral transplants. Brain Res. 1979;177:555–560. - PubMed
-
- Borlongan CV, Tajima Y, Trojanowski JQ, Lee VM, Sanberg PR. Transplantation of cryopreserved human embryonal carcinoma-derived neurons (nt2n cells) promotes functional recovery in ischemic rats. Exp Neurol. 1998;149:310–321. - PubMed
-
- Kordower JH, Freeman TB, Snow BJ, Vingerhoets FJ, Mufson EJ, Sanberg PR, Hauser RA, Smith DA, Nauert GM, Perl DP, Olanow CW. Neuropathological evidence of graft survival and striatal reinnervation after the transplantation of fetal mesencephalic tissue in a patient with parkinson’s disease. N Engl J Med. 1995;332:1118–1124. - PubMed
-
- Kondziolka D, Wechsler L, Goldstein S, Meltzer C, Thulborn KR, Gebel J, Jannetta P, DeCesare S, Elder EM, McGrogan M, Reitman MA, Bynum L. Transplantation of cultured human neuronal cells for patients with stroke. Neurology. 2000;55:565–569. - PubMed
-
- Bang OY, Lee JS, Lee PH, Lee G. Autologous mesenchymal stem cell transplantation in stroke patients. Ann Neurol. 2005;57:874–882. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

