Subversion of CtBP1-controlled macropinocytosis by human adenovirus serotype 3
- PMID: 18323776
- PMCID: PMC2323250
- DOI: 10.1038/emboj.2008.38
Subversion of CtBP1-controlled macropinocytosis by human adenovirus serotype 3
Abstract
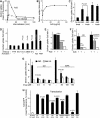
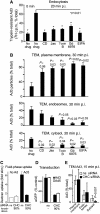
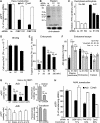
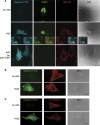
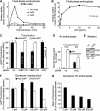
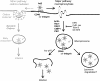
Endocytosis supports cell communication, growth, and pathogen infection. The species B human adenovirus serotype 3 (Ad3) is associated with epidemic conjunctivitis, and fatal respiratory and systemic disease. Here we show that Ad3 uses dynamin-independent endocytosis for rapid infectious entry into epithelial and haematopoietic cells. Unlike Ad5, which uses dynamin-dependent endocytosis, Ad3 endocytosis spatially and temporally coincided with enhanced fluid-phase uptake. It was sensitive to macropinocytosis inhibitors targeting F-actin, protein kinase C, the sodium-proton exchanger, and Rac1 but not Cdc42. Infectious Ad3 macropinocytosis required viral activation of p21-activated kinase 1 (PAK1) and the C-terminal binding protein 1 of E1A (CtBP1), recruited to macropinosomes. These macropinosomes also contained the Ad3 receptors CD46 and alpha v integrins. CtBP1 is a phosphorylation target of PAK1, and is bifunctionally involved in membrane traffic and transcriptional repression of cell cycle, cancer, and innate immunity pathways. Phosphorylation-defective S147A-CtBP1 blocked Ad3 but not Ad5 infection, providing a direct link between PAK1 and CtBP1. The data show that viruses induce macropinocytosis for infectious entry, a pathway used in antigen presentation and cell migration.
Figures



References
-
- Aktories K (1997) Rho proteins: targets for bacterial toxins. Trends Microbiol 5: 282–288 - PubMed
-
- Barnes CJ, Vadlamudi RK, Mishra SK, Jacobson RH, Li F, Kumar R (2003) Functional inactivation of a transcriptional corepressor by a signaling kinase. Nat Struct Biol 10: 622–628 - PubMed
-
- Baumgartner M, Patel H, Barber DL (2004) Na(+)/H(+) exchanger NHE1 as plasma membrane scaffold in the assembly of signaling complexes. Am J Physiol Cell Physiol 287: C844–C850 - PubMed
-
- Benmerah A, Bayrou M, Cerf-Bensussan N, Dautry-Varsat A (1999) Inhibition of clathrin-coated pit assembly by an Eps15 mutant. J Cell Sci 112: 1303–1311 - PubMed
-
- Berk AJ (2005) Recent lessons in gene expression, cell cycle control, and cell biology from adenovirus. Oncogene 24: 7673–7685 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

