Transgenesis and neuronal ablation in parasitic nematodes: revolutionary new tools to dissect host-parasite interactions
- PMID: 18324923
- PMCID: PMC3086006
- DOI: 10.1111/j.1365-3024.2008.01006.x
Transgenesis and neuronal ablation in parasitic nematodes: revolutionary new tools to dissect host-parasite interactions
Abstract
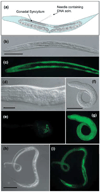
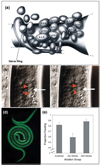
Ease of experimental gene transfer into viral and prokaryotic pathogens has made transgenesis a powerful tool for investigating the interactions of these pathogens with the host immune system. Recent advances have made this approach feasible for more complex protozoan parasites. By contrast, the lack of a system for heritable transgenesis in parasitic nematodes has hampered progress toward understanding the development of nematode-specific cellular responses. Recently, however, significant strides towards such a system have been made in several parasitic nematodes, and the possible applications of these in immunological research should now be contemplated. In addition, methods for targeted cell ablation have been successfully adapted from Caenorhabditis elegans methodology and applied to studies of neurobiology and behaviour in Strongyloides stercoralis. Together, these new technical developments offer exciting new tools to interrogate multiple aspects of the host-parasite interaction following nematode infection.
Figures

Similar articles
-
Transgenesis in Strongyloides and related parasitic nematodes: historical perspectives, current functional genomic applications and progress towards gene disruption and editing.Parasitology. 2017 Mar;144(3):327-342. doi: 10.1017/S0031182016000391. Epub 2016 Mar 22. Parasitology. 2017. PMID: 27000743 Free PMC article. Review.
-
Terror in the dirt: Sensory determinants of host seeking in soil-transmitted mammalian-parasitic nematodes.Int J Parasitol Drugs Drug Resist. 2018 Dec;8(3):496-510. doi: 10.1016/j.ijpddr.2018.10.008. Epub 2018 Oct 26. Int J Parasitol Drugs Drug Resist. 2018. PMID: 30396862 Free PMC article. Review.
-
The Use of Caenorhabditis elegans as a Model for Plant-Parasitic Nematodes: What Have We Learned?Annu Rev Phytopathol. 2024 Sep;62(1):157-172. doi: 10.1146/annurev-phyto-021622-113539. Epub 2024 Aug 22. Annu Rev Phytopathol. 2024. PMID: 38848590 Review.
-
Rendering the Intractable More Tractable: Tools from Caenorhabditis elegans Ripe for Import into Parasitic Nematodes.Genetics. 2015 Dec;201(4):1279-94. doi: 10.1534/genetics.115.182717. Genetics. 2015. PMID: 26644478 Free PMC article. Review.
-
Elucidating the molecular and developmental biology of parasitic nematodes: Moving to a multiomics paradigm.Adv Parasitol. 2020;108:175-229. doi: 10.1016/bs.apar.2019.12.005. Epub 2020 Jan 31. Adv Parasitol. 2020. PMID: 32291085 Review.
Cited by
-
Microarray-based analysis of differential gene expression between infective and noninfective larvae of Strongyloides stercoralis.PLoS Negl Trop Dis. 2011 May 3;5(5):e1039. doi: 10.1371/journal.pntd.0001039. PLoS Negl Trop Dis. 2011. PMID: 21572524 Free PMC article.
-
Recent advances in functional genomics for parasitic nematodes of mammals.J Exp Biol. 2020 Feb 7;223(Pt Suppl 1):jeb206482. doi: 10.1242/jeb.206482. J Exp Biol. 2020. PMID: 32034038 Free PMC article. Review.
-
Glutamate-gated chloride channels of Haemonchus contortus restore drug sensitivity to ivermectin resistant Caenorhabditis elegans.PLoS One. 2011;6(7):e22390. doi: 10.1371/journal.pone.0022390. Epub 2011 Jul 26. PLoS One. 2011. PMID: 21818319 Free PMC article.
-
piggyBac: A vehicle for integrative DNA transformation of parasitic nematodes.Mob Genet Elements. 2013 Mar 1;3(2):e24417. doi: 10.4161/mge.24417. Mob Genet Elements. 2013. PMID: 23914309 Free PMC article.
-
Phenotypic screen of early-developing larvae of the blood fluke, schistosoma mansoni, using RNA interference.PLoS Negl Trop Dis. 2009 Aug 11;3(8):e502. doi: 10.1371/journal.pntd.0000502. PLoS Negl Trop Dis. 2009. PMID: 19668375 Free PMC article.
References
-
- Dzierszinski FS, Hunter CA. Advances in the use of genetically engineered parasites to study immunity to Toxoplasma gondii. Parasite Immunol. 2008;30:235–244. - PubMed
-
- Mansfield JM, Paulnock DM. Genetic manipulation of African trypanosomes as a tool to dissect the immunobiology of infection. Parasite Immunol. 2008;30:245–253. - PubMed
-
- Thompson J, Millington OR, Garside P, Brewer JM. What can transgenic parasites tell us about the development of Plasmodium-specific immune responses? Parasite Immunol. 2008;30:223–233. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

